Current options for tubular tissue reconstruction are extremely limited—particularly in pediatrics
Long segmental tracheal defects present a significant reconstructive challenge. In adults, only lesions encompassing less than 50% of the tracheal length can be reconstructed with end-to-end anastomosis. In children, only defects covering less than 40% of the tracheal length can be reconstructed. These lengths may be further reduced by previous interventions, such as multiple operations that can cause scarring or radiation that can reduce tracheal blood supply. Attempting to create longer segments through end-to-end anastomosis may cause significant tension at the anastomosis site and lead to local necrosis, leakage, and potentially stricture and stenosis. In children, if the implanted device cannot accommodate the normal longitudinal and radial growth of tubular tissues after implantation, it may eventually restrict growth.
Versatile splinting device enables long-passageway reconstruction in growing children and adults
This innovation advances splinting devices by applying functional patterns for passageways or tubular tissues (e.g., trachea, bronchi, esophagus, nerves, blood vessels) to potentially enable long-passageway reconstructions currently not possible. It also has the potential to address growth in pediatric patients who have undergone reconstruction. The stent-patterned device can be used to externally support long-passageway defects or as a framework for pre-vascularized tubular tissue flaps during reconstruction of long-passageway defects after resection.
While the application of the stent-pattern that creates longitudinal bending flexibility decreases the radial rigidity of the device, the rigidity issue is addressed by either increasing the wall thickness or using an extrusion-based 3D-printing method while maintaining the same wall thickness. When this stent-patterned device is used as a framework for the pre-vascularized tubular tissue flap, it has a core post in the luminal area that is connected to the device with multiple thin bridges. The core post is easily removable after successful soft tissue ingrowth. This device can also be combined with hydrogel-based biomaterials, with or without growth factors, to enhance vascular and tissue growth.
Incorporating auxetic-patterning creates an implantable splinting device for supporting passageway defects in growing patients, such as infants or children. The auxetic patterns allow the device to expand along a radial direction as the longitudinal length increases, corresponding to the normal growth of the passageway. These auxetic-patterned splinting devices can be placed around any patient passageway including, but not limited to, the trachea, bronchi, esophagus, nerves, and blood vessels.
- Enables longer scaffolds: As an airway support device, this device can address longer stenotic segments than previous devices because stent patterns on the wall give the device bending flexibility.
- Increases mechanical strength: Using extrusion-based 3D bioprinting produces a patterned implantable device with higher mechanical strength than the methods that use selective laser sintering 3D printing, without increasing the wall thickness of the device.
- Enhances stability: Incorporating various biomaterials enables the device to be customized to suit the mechanical properties and behavior of the targeted tubular organs. This helps assure stability and open passageways without interfering with the actual movement of the long passageways.
- Promotes vascularization and tissue growth: Incorporating hydrogels—collagen, hyaluronic acid, and decellularized extracellular matrix (both with and without growth factors)—supports growth of new blood vessels and tissue.
- Addresses pediatric needs: When using auxetic patterns, the splinting device corresponds to the normal growth of the passageway of infants or children. It not only expands in the radial direction with incremental increases in the longitudinal direction but also extends in the longitudinal direction with expansion of the radial direction.
Patterned implantable devices for placement in passageways, such as the trachea, bronchi, esophagus, nerves, and blood vessels, in humans and animals.
- Long-segment tracheal reconstruction
- Auxetic splinting devices (particularly for pediatric patients)
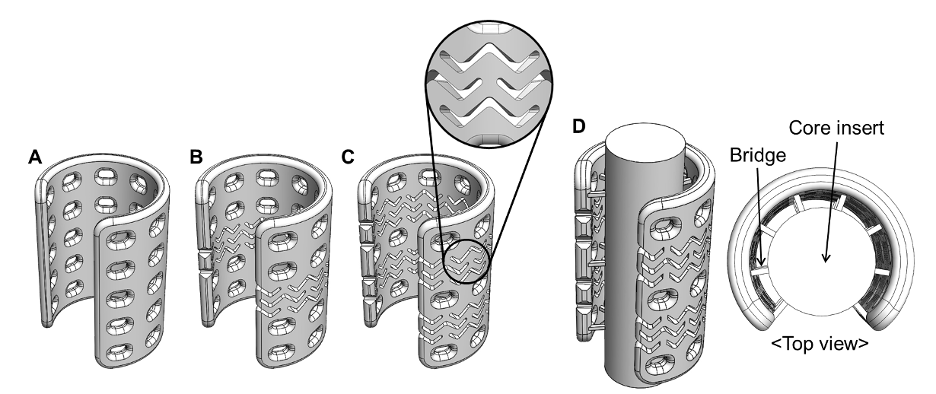
Design of the airway scaffolds. (A) Airway scaffold without stent-pattern. (B) Airway scaffold with a stent-pattern. (C) Airway scaffold with two stent-patterns. (D) The two stent-patterned airway scaffold with the core insert to create the tubular tissue flap in vivo.
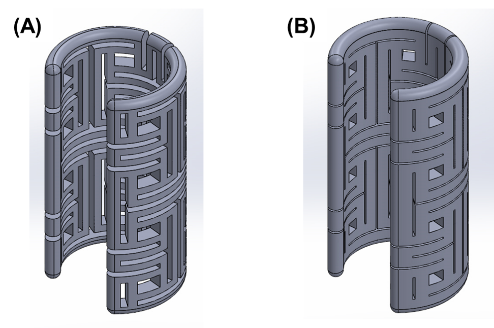
The auxetic-patterned implantable device of 2-mm wall thickness, showing different line widths and intervals.
