Low sensitivity of current lateral flow assays results in high rates of false negatives
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to threaten daily life by evolving into new variants with greater transmissibility. Although lateral flow assays (LFAs) are widely used to self-test for coronavirus disease 2019 (COVID-19), these tests suffer from low sensitivity, leading to a high rate of false-negative results. The inherent low sensitivity of current tests necessitates more invasive specimen collection, such as nasal or nasopharyngeal swabs, instead of more non-invasive collection of specimens, such as saliva. This unpleasant sampling method leads to testing reluctance and less frequent self-testing, which ultimately defeats the purpose of early detection to minimize transmission.
Additionally, existing paper-based diagnostic tests lack the ability to manipulate the capillary-driven flow. This makes it difficult and impractical to perform multistep bioassays that require timely application of reagents or buffers in multiple steps, such as immunoassay, enzyme-linked immunosorbent assay (ELISA), and sample purification.
Practical and scalable manipulation of capillary-driven flow enables significantly higher test sensitivity
This simple, scalable method enables tunable capillary flow on paper using imprinted timers and has the potential to make traditionally labor-intensive assays available in simple paper formats.
A tunable delaminating timer with asymmetric design creates a fluidic diode and transistor. These timers are imprinted at strategic nodes using water-insoluble inks to produce the required flow delay that occurs via the gradual delamination between the ink-infused paper and the laminating tape. By using sets of inks and tapes with varying material properties, a custom tunable flow delay can be created, ranging from tens of seconds to minutes or even hours. Coordinating different capillary flows to sequentially introduce different reagents into a reaction, with optimal incubation times in between, enables the autonomous use of complex assays that are otherwise not possible with conventional LFAs.
By incorporating chemically amplified multiplex detection, this method has detected SARS-CoV-2 and influenza A and B viruses with ~25x higher sensitivity than commercial LFAs. It has also detected positive SARS-CoV-2 patient saliva samples in tests missed by commercial LFAs.
This innovation has the potential to transform a variety of biological assays and tests exclusively performed at clinical laboratories into single-use, disposable dipstick tests to be used at the point of care or home. These tests could rival ELISA or polymerase chain reaction (PCR) assays for sensitive and specific detection of pathogenic targets, such as Zika virus, human immunodeficiency virus (HIV), hepatitis B virus, or diseases like malaria.
- Reduces false negatives: Compared to commercial LFAs for COVID-19 testing, this device achieves enhanced sensitivity even with the use of the same concentration of the sample, reducing the risk of false negatives.
- Enables multiplexed assays: Tunable capillary flow allows sample/reagent to be concurrently delivered to multiple regions, enabling multiplexed and repetitive assays on a single device.
- Improves point-of-care testing: Transforms a variety of biological assays and tests exclusively performed at clinical laboratories into single-use, disposable dipstick tests to be used at the point of care or home.
- Simplifies use: The LFA with built-in signal amplification can both run an assay and amplify the result by simply adding the sample. The process is automatically handled by the device, and the results are observed as color changes in the test lines.
- Reduces sample volume: Sample volume can be minimized, which is particularly important for volume-limited samples, such as tears, saliva, sweat, urine, and blood from infants.
- Eliminates potential artifacts: A built-in wash step quenches the reaction and eliminates potential artifacts from delayed observation of the colorimetric results, which helps produce consistent readings and ensure against false-positive readings.
To enhance point-of-care and self-testing of:
- SARS-CoV-2
- Influenza A and B
- Zika virus
- HIV
- Hepatitis B
- Malaria
Other sensor applications:
- Environmental/Food safety
- Biomedicine for diagnostic, therapeutic, and research purposes
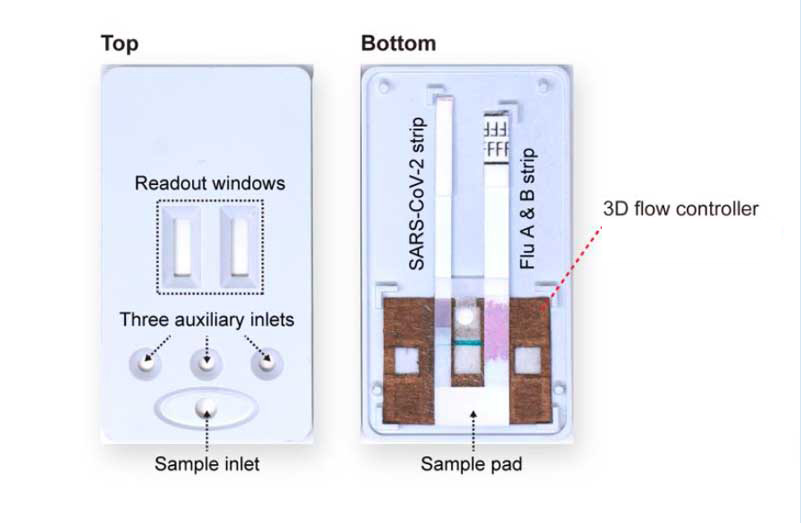
The device fully assembled in the 3D-printed case (left) and with its front cover removed (right). The back cover holds the internal components: commercial LFA strips of SARS-CoV-2 and influenza A and B and the paint-programmed paper-based flow controller.
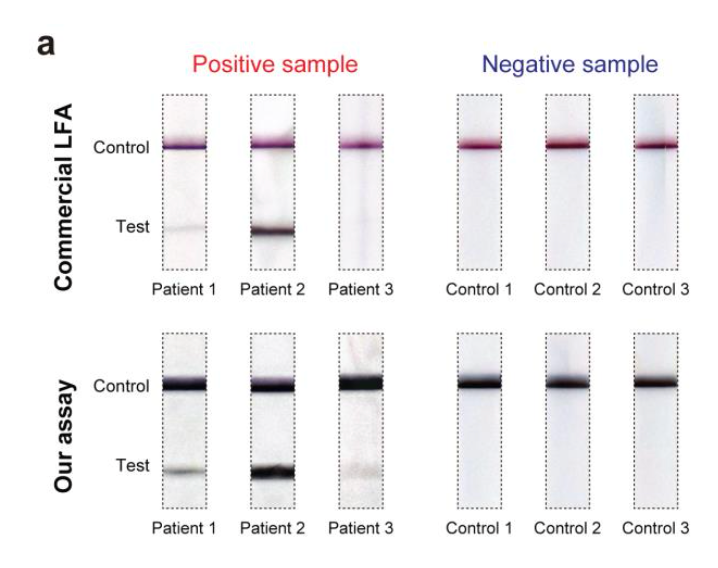
Close-up photos of the test and control lines of a commercial COVID-19 test strip (top) and this device (bottom) after they were used to process matched (left) saliva samples from three COVID-19 patients and (right) control samples collected from three healthy donors. Dark control lines along the top of all strips show the detection process was performed correctly. A lack of test lines in the control shows no false positives. The test section for three COVID-19 patients shows no false negatives for the Georgia Tech assay, while patient 3 tested negative using the commercial LFA.
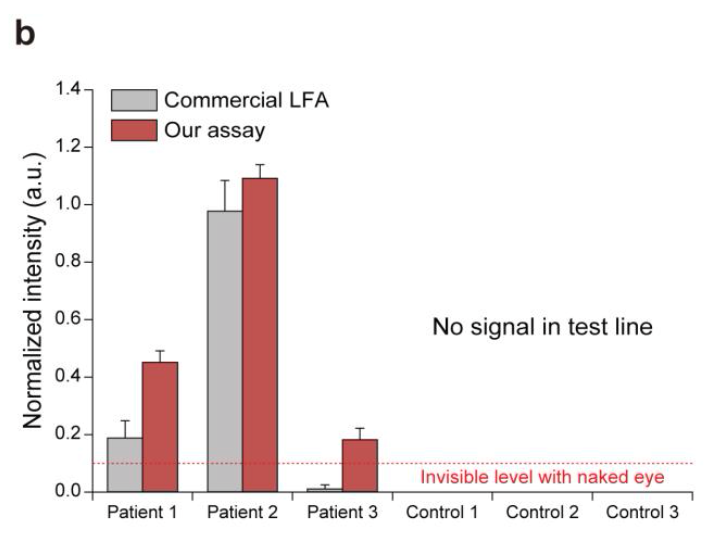
A plot showing the normalized intensities measured at the test lines of a commercial LFA (grey bars, left) and this assay (red bars, right) for processed patient and control samples. The colorimetric signal level that could not be seen via the naked eye is marked on the plot.
