Current ex vivo organoid models are unable to recapitulate spatiotemporal patterning of immune cells
B lymphocytes and T lymphocytes play an essential role in protecting a host against pathogens. They go through several complex interactions to form germinal centers (GCs) where B cells can proliferate, increase antibody affinity, and differentiate into either memory B cells or long-lived plasma cells.
Ex vivo organoid models have accelerated our understanding of physiological, molecular, and cellular phenomenon and can support the study of GC formation and antigen response. However, the interactions that play a role in GC formation—including transcriptomic and epigenetic reprograming, extracellular matrix remodeling, and cell-cell interactions—are complex, which limits the models that can show GC formation in human lymphoid follicles.
The established maleimide-functionalized poly(ethylene glycol) (PEG-MAL) hydrogel is an excellent biomaterial for primary immune cell survival and differentiation, but it is unable to recapitulate spatiotemporal patterning of immune cells as is seen in vivo. Its fast crosslinking prevents PEG-MAL patterning in complex microfluidic tissue/organ-on-a-chip and related devices. Alternative PEG polymers, such as vinyl sulfones and acrylates, do not support primary immune cell differentiation or survival in vitro. Furthermore, synthetic polymers as well as natural matrices (e.g., collagen, murine tumor matrix extract) are very challenging to pattern at the microscale to direct cellular activities.
Gain better spatiotemporal control and customization of hydrogel crosslinking and cell patterning
Unlike PEG-MAL hydrogels, norbornene functionalized PEG (PEG-NB) hydrogels are crosslinked using a photoinitiated reaction with thiol groups. This reaction, through localization to the irradiated area, supports better spatiotemporal control of crosslinking and patterning. It represents a viable alternative to PEG-MAL by enabling facile cell patterning and the study of cell responses to antigens as well as culturing primary B cells ex vivo.
With this innovation, complex patterns (including biomolecular, cellular, and chemical gradients) can be generated in a synthetic hydrogel system using spatial patterning of the polymer network, proteins, multiple immune and non-immune cell types, and other non-cellular entities. This approach reduces timing and compatibility issues and offers designer flexibility compared to natural matrices.
- Customizable design: Using PEG-NB, this innovation results in highly tunable properties, including those that are structural, biophysical, and biochemical as well as degradation, polymeric and protein gradients, cell-based gradients.
- Increased compatibility: This approach offers designer flexibility comparable to natural matrices while (a) reducing the timing issues found with device injectability and crosslinking of PEG-MAL and (b) enhancing the immune cell compatibility found with other polymeric formulations like vinyl sulfone-PEG.
- High throughput with high fidelity: This approach enables the use of high-throughput microfluidic devices to study immune cells in a patterned space that mimics natural interactions.
PEG-NB can be used as an alternative to PEG-MAL for developing synthetic immune tissues in microfluidic devices for various applications, including:
- Diagnostics
- Disease modeling
- Regenerative medicine
- Immunotherapy
- Immunogenicity
- Drug screening
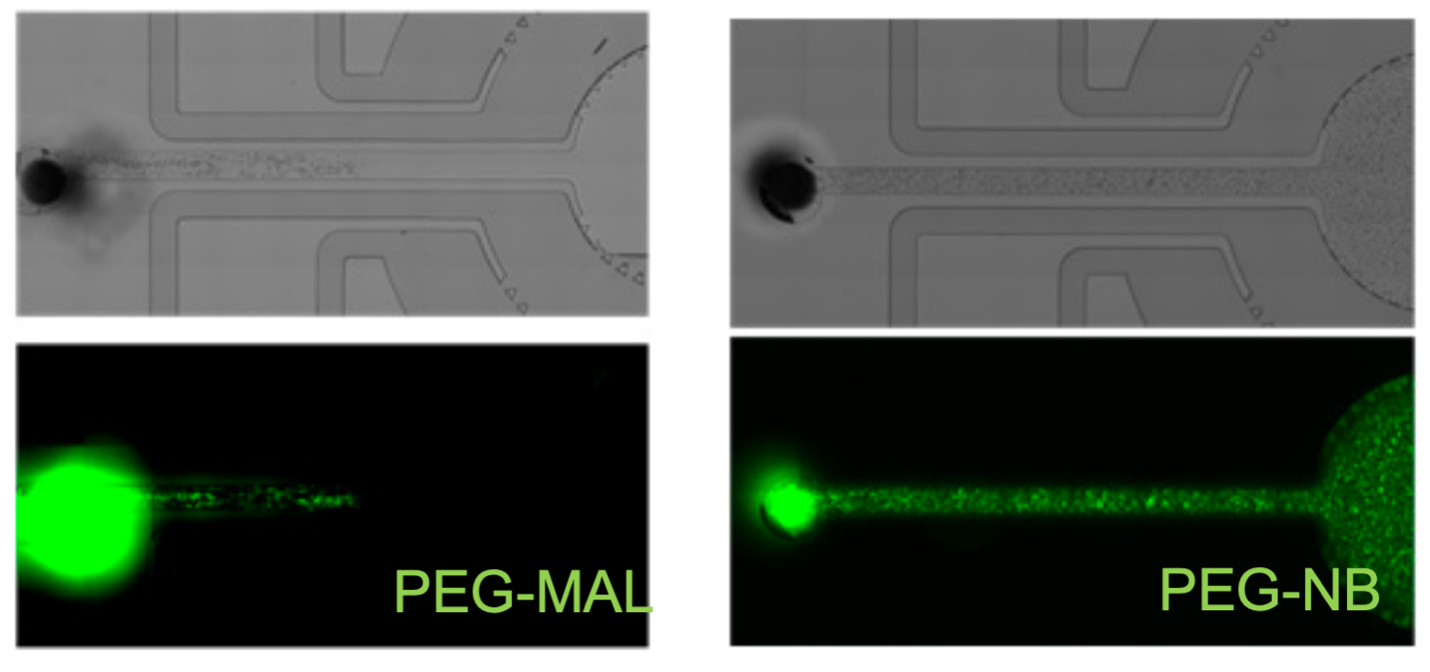
(left) Illustrates PEG-MAL crosslinking chemistry not supporting injectability or patterning in microfluidic devices. (right) Illustrates PEG-NB photoinitiated chemistry allowing cell patterning and injectability in microfluidic devices.
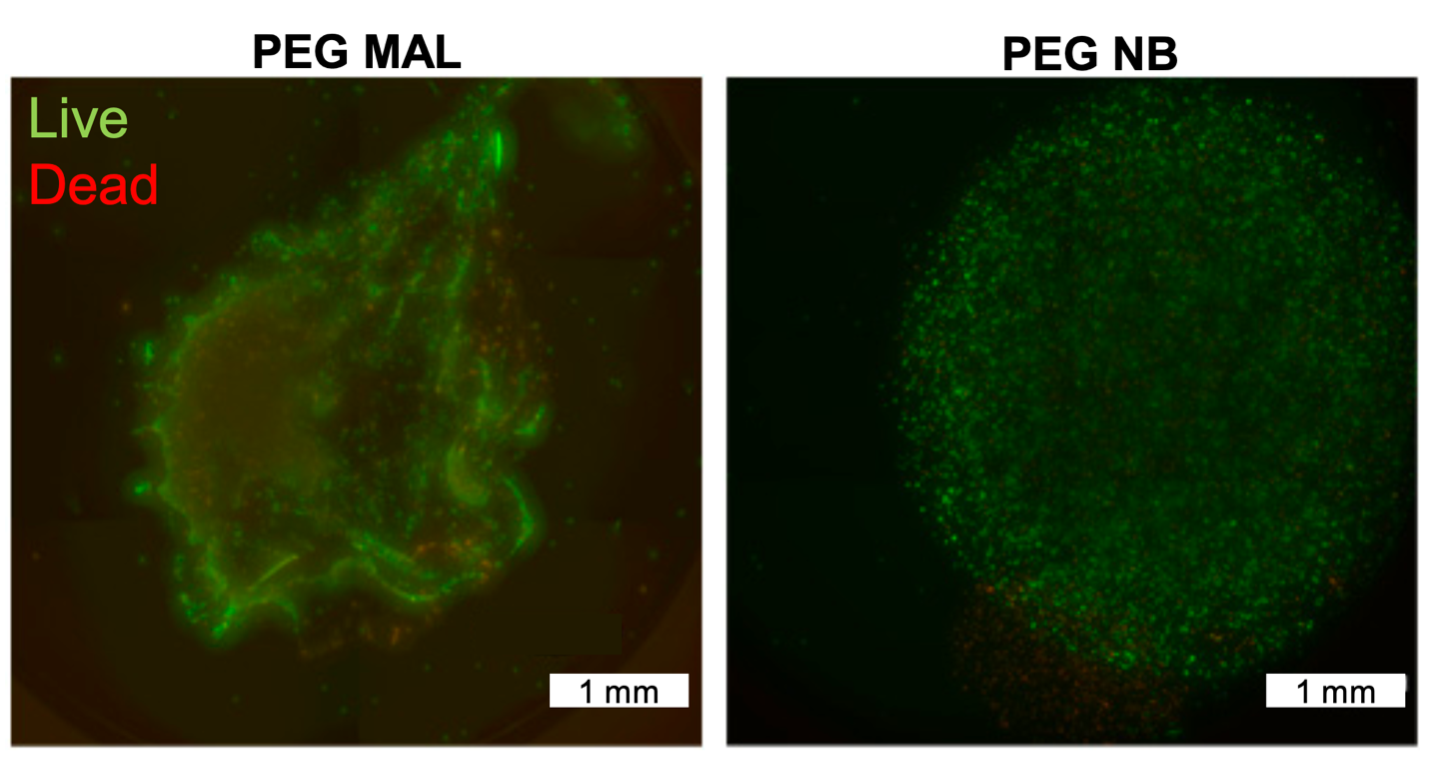
Both PEG-MAL and PEG-NB hydrogels shown encapsulating mammalian B cells; however, PEG-NB shows a higher homogenous cell distribution.
