State-of-the-art engineered immune tissues lack spatial and temporal capabilities
Antibody-based immunotherapy is on the rise in the treatment of multiple pathological conditions such as infections, cancer, and autoimmune diseases. Patients affected by these disorders often have impaired immune responses resulting in poor clinical outcomes.
Engineered immune tissues are currently used in immunotherapy but lack the ability to direct spatial and temporal immune cell differentiation in response to stimuli, leading to an ineffectual immune reaction and therapeutic outcome. Harnessing immune reactions, in a controlled, ex vivo environment, would create the opportunity to reproduce immunologic events with controllable parameters and enable novel therapeutic development and approaches.
Engineered tissue system may enable better therapeutics through reproducible, controllable, ex vivo immunologic events
This system combines advanced biomaterials, microfluidics-based chemokine gradients, and lymphoid tissue biophysical properties, along with primary human immune cells. Microfluidic-devices are used to develop the gradient of immune cells, attracting chemokine across a synthetic, hydrogel-based, lymphoid microenvironment. This system uniquely demonstrates controlled modulation of various immune cells via the switchable nature of the protein gradient. This particular innovation allows immature immune cells to differentiate appropriately to enable the study of the immune response by recapitulating complex immunological events, such as germinal center (GC) reactions and differentiation of B cells, in a user-controlled manner. Additionally, the ex vivo medium supports creating an immune response in a controlled environment, allowing reproduction of immunologic events that would inform therapeutic development, reduce vaccine development times and costs, improve vaccine efficacy, and reduce reliance on animal models.
- Controllable: Using an ex vivo environment, this technology is designed to enable reproducible immunologic responses with manageable parameters for quick antibody generation and B cell dysfunction modeling.
- Switchable: The chemokine gradient is switchable, allowing the B cell differentiation required for an appropriate immune reaction.
- Tested: The system has provided insights into the impaired GC response against influenza vaccines in survivors of diffuse large B cell lymphoma patients
This technology enables recapitulation of immune responses for the study of:
- Disease, cancer, and infection
- Multi-organ immunologic integration
- Vaccine and therapeutic development
- Disease transmission and pathology research
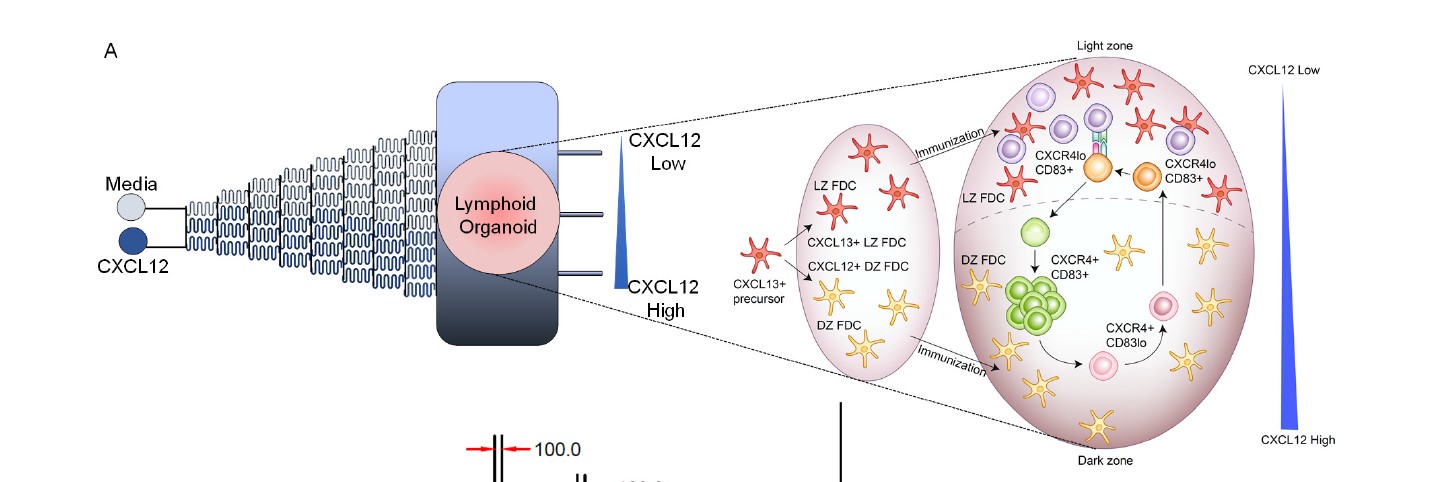
Schematic of the lymphocyte-encapsulated tissues with switchable gradients in organ-on-chip exhibiting device design and underlying biology.
