Human umbilical cord tissue mesenchymal stromal cells (hCT-MSCs) have been widely evaluated in several clinical trials for treating patients with a variety of medical conditions, including COVID-19. There are, however, significant challenges related to their manufacturing, and biological variabilities remain. A key challenge in cell therapy manufacturing is understanding how the bioreactor environment affects cell proliferation and quality. Additionally, there are no current strategies for implementing automated feedback controls to improve process efficiency and product reproducibility.
Current state-of-the-art bioreactors rely on off-line sampling and manual adjustment of feed rates in their expansion protocols. This fixed recipe for culturing cannot account for variabilities in the expansion process, as is often the case with unique donor cells. Optimizing these large-scale bioreactors is also limited by resources and cost.
Next-generation bioreactor provides controls for critical process parameters to maintain a consistent, scalable, and reproducible growth environment for cells
This “smart” bioreactor platform is a semi-autonomous system that incorporates in-line sensors, modeling, data-driven controllers, and an automated sampling platform.
Its feedback-controlled hollow fiber-based bioreactor enables identifying critical quality attributes (CQA) and critical process parameters (CPP) for high-quantity and high-quality hCT-MSCs for ideal cell growth and efficacy. It also provides the controls to maintain these conditions to improve expansion yield and cell viability. Based on large-scale commercial bioreactors, this scaled-down system leverages sensors and state estimation models to inform and control CPPs and maintain a more consistent growth environment for cells that is both scalable and reproducible. The small-scale system reduces costs, labor, time, and perturbations and improves yields of MSC products.
The design paves the way for a next-generation bioreactor that permits plug-and-play integration of analytical technologies to shape the biomanufacturing landscape for cell therapies and to potentially enable the wide-spread use of MSCs as a clinical therapy.
- Reduces costs: Improved efficiency and automation minimizes associated expenses (e.g., costs for materials, labor, and time)
- Improves yield and viability: Feedback controls enable identifying the CQA and CPP for high-quantity and high-quality hCT-MSCs and maximizes the functional quality (therapeutic potency), yields, reproducibility, and reliability
- Refines control: In-line sensors with automated feedback controls and automated sampling increases control of critical cell-specific parameters
- Enhances precision: Automated sampling process reduces risks (e.g., contamination) and bioreactor perturbations, while improving results and enhancing precision
- Simplifies production: Platform includes hardware and software for extracting samples and storing in vials for off-line analysis and is controlled through a graphical interface that enables taking multiple samples at fixed intervals throughout the duration of the expansion
- Cell therapy manufacturing for therapeutic (e.g., hCT-MSCs for wide-spread use as a clinical therapy) and research uses
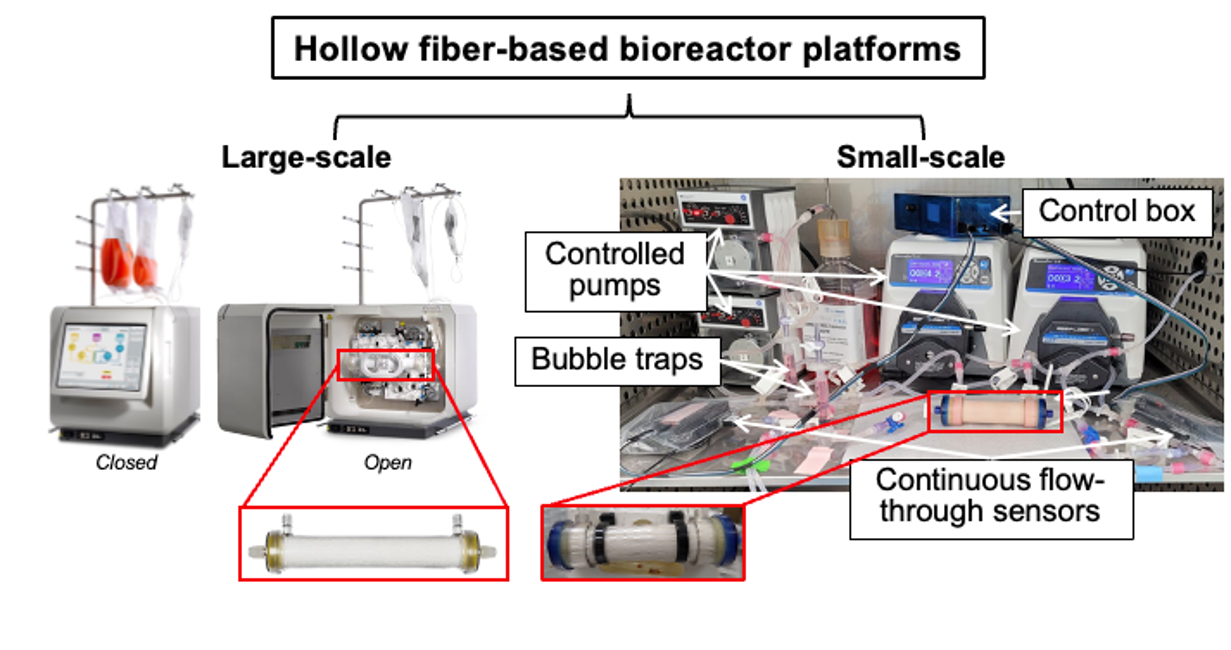
“Smart” hollow fiber bioreactor with in-line sensors informing feedback controls for autonomous feeding and regulation of culture environment.
