HO technology provides a powerful platform for the functional modeling and repair of genetic defects in human development and in the establishment of chronic disease models, such as inflammatory bowel disease. Current methods for generating HOs rely on growth in a complex, poorly defined, tumor-derived extracellular matrix (ECM). This severely limits their use in regenerative medicine and clinical translation and restricts scale-up applications in drug testing and patient applications. The limitations are caused by the lot-to-lot variations in their composition and structure due to their tumor-derived nature and the regulatory barriers that come with the matrix source (mouse tumor cells).
New fully synthetic hydrogels generate HOs in vitro and support in vivo HO delivery, promoting engraftment and function
This fully synthetic hydrogel offers in vitro generation of organoids, including intestinal and lung organoids, derived from human pluripotent stem cells (hPSC) without the use of natural ECM-based media. This optimal formulation supports hPSC survival, expansion, and epithelial differentiation into intestinal HOs as well as differentiation into mature intestinal tissue in vivo at levels similar commercially available matrices. The synthetic matrix also has potential for use in the development of other HOs.
- Improved HO viability: Based on a four-arm poly(ethylene glycol) (PEG) macromer with maleimide groups at each terminus (PEG-4MAL), this synthetic hydrogel exhibits high cytocompatibility and minimal toxicity and inflammation in vivo.
- Greater control: PEG-4MAL hydrogel allows control over the matrix composition and its physiochemical properties, while also providing control over gelling time which enables components to be injected as a solution that gels in situ.
- Enhanced wound repair: Injectable delivery vehicle supports localized HO engraftment in mucosal wounds to potentially enhance wound closure and repair.
- Supports HO development: Engineered PEG-4MAL hydrogel robustly supports HO development and maintenance, with cultures showing the same growth, shape, central lumen, and epithelial budding at the interface with the hydrogel as with commercial matrices.
- Supports regenerative medicine: Modular design and ability to deliver via endoscopic techniques creates the translational potential of this delivery platform for regenerative medicine and overcomes limitations associated with the use of commercially available matrices for hPSC-based organoid technologies.
- Regenerative medicine therapeutic for treating intestinal injury or disease
- In vitro generation of human organoids (HOs) and subsequent in vivo delivery of HOs for regenerative medicine
- Research tool for drug screening applications and diseased models using HOs
- Research tool to enable further investigation of human intestinal organoids (HIO) delivery-based intestinal tissue regeneration and resolution of intestinal disease in murine models
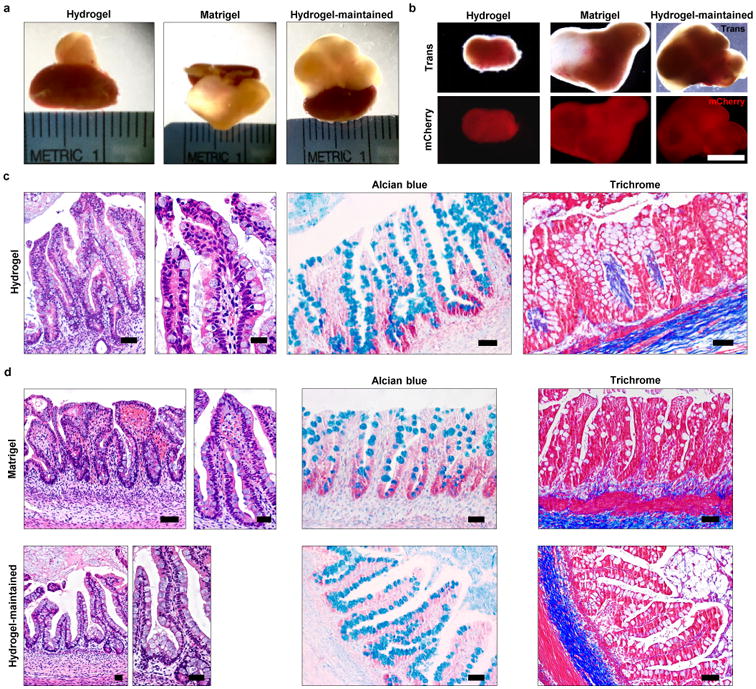
Figure 1. PEG-4MAL-generated HIOs develop a mature intestinal tissue structure in vivo (a) Micrographs of dissected kidneys containing HIOs generated within PEG-4MAL hydrogel, Matrigel™, or generated within Matrigel™ and maintained within PEG-4MAL (hydrogel-maintained) (b) Transmitted light and fluorescence microscopy (mCherry) images of harvested organoids (c,d) Hematoxylin and eosin staining demonstrates mature human intestinal crypt-villus structure, and Alcian blue and trichrome staining reveal presence of differentiated goblet cells and organized collagen fibers.
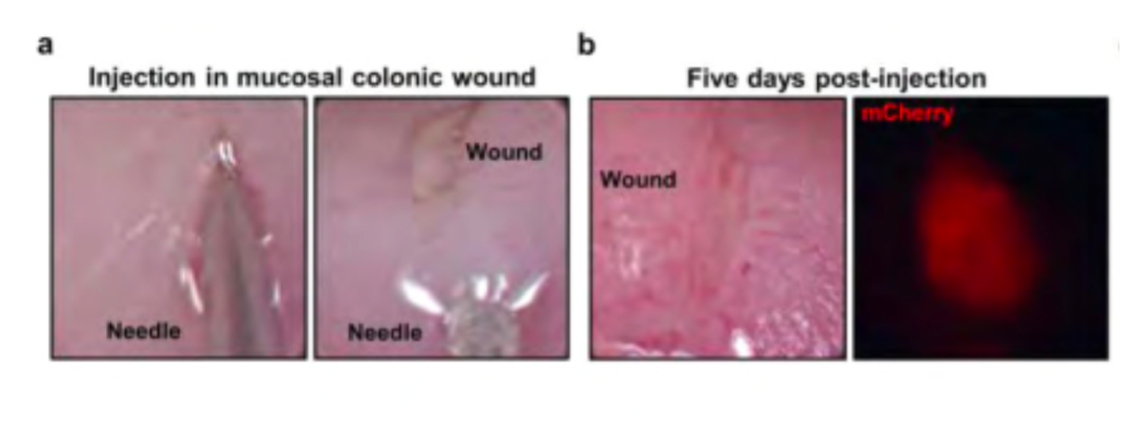
Figure 2. (a) PEG-4MAL-generated HIOs mixed with engineered hydrogel precursor solutions being injected underneath mechanically induced mucosal wounds (b) The mucosal wound five days post-injection
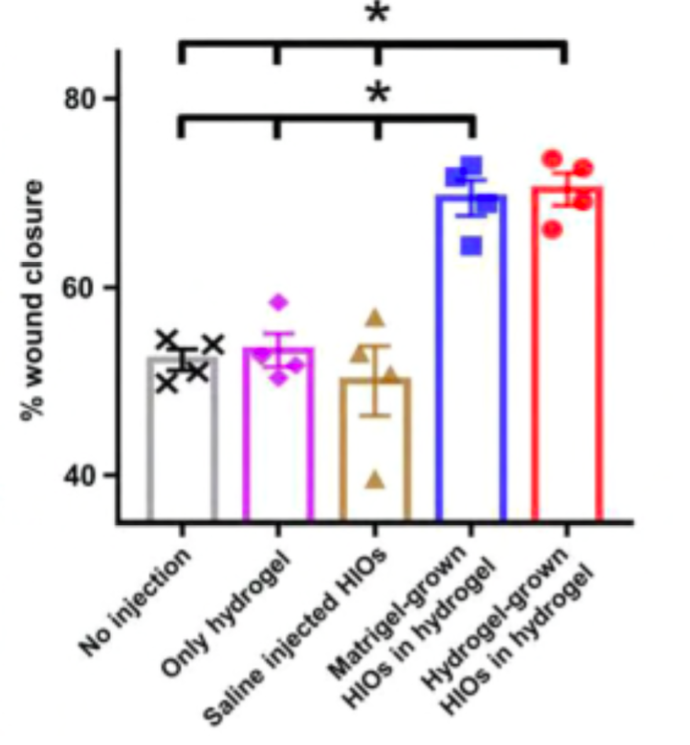
Figure 3. Comparing wound closure results after injection of Matrigel™-grown HIOs in hydrogel to Hydrogel-grown HIOs in hydrogel
