Manually reprogramming stem cells is inefficient and time-consuming
Undifferentiated human induced pluripotent stem cells are capable of producing a wide range of other cell types. Reprogramming these cells from a patient’s body may make it possible to develop regenerative therapies for disorders or diseases such as Alzheimer’s, Parkinson’s, or diabetes. However, cell reprogramming techniques are inefficient and time-consuming, which limits the use of hiPSCs in research and therapeutic development. Current techniques require manually isolating the pluripotent stem cells from a large mixture of cells in order to maintain hiPSC survival, purity, and karyotype stability. This process requires a level of skill that limits use of the technique, creates a bottleneck for hiPSC purification, and slows the development of potential therapies. An automated, high-throughput process is needed that eliminates manual separation, enzymatic passaging, and labeling with antibodies or other reagents.
Tunable microfluidic-based process separates intact cell colonies quickly and efficiently
Based on the distinct adhesive signatures of human pluripotent stem cells, this tunable high-throughput process separates cells quickly and efficiently according to the degree to which they adhere to a substrate inside the microfluidic device. The system uses controllable fluid forces to isolate hiPSCs from parental fibroblasts and spontaneously differentiated hiPSCs, while also maintaining the normal transcriptional profiles, differentiation potential, and karyotype. The hiPSCs’ adhesion properties differ significantly from those of the cells with which they are mixed, potentially enabling therapeutic cells to be separated with as much as 99% purity.
This simple microfluidic-based approach is fast (< 10 minutes to perform), does not rely on labeling technologies (such as antibodies), supports greatly enriched cells (650-fold compared to the initial culture), and results in a cell survival rate greater than 80%. By allowing separation of intact cell colonies, this simple system avoids damaging and adversely affecting the pluripotency of the cells.
- Scalable: This technology allows standardization across laboratories with consistent results and is amenable to high-throughput analyses; real-time imaging; and in-line biochemical, genetic, and cytometric processing.
- Fast: Its high-throughput process takes less than 10 minutes to perform.
- Higher cell survival: Cells are isolated as colonies instead of single cells to improve cell survival.
- Simple: This automated system does not require the ability to morphologically recognize undifferentiated cells and, therefore, does not depend on users’ skill level.
- Versatile: The technique can be used with all adhesive force-based detachment methods, including hydrodynamic flow, centrifugation, electromagnetism, and micromanipulation. It is applicable to both hiPSCs and human embryonic stem cells.
- Cost-effective: This easy-to-implement system reduces costs with automation and speed.
- Advances therapies: It has the potential to improve modeling techniques to enable advanced therapies.
This new technique has wide applications in basic research and regenerative therapeutic development. It is applicable to the selective isolation and purification of pluripotent stem cells to:
- Expand production of stem cells generated through cell reprogramming
- Improve current remodeling techniques and disease process modeling
- Develop regenerative therapies
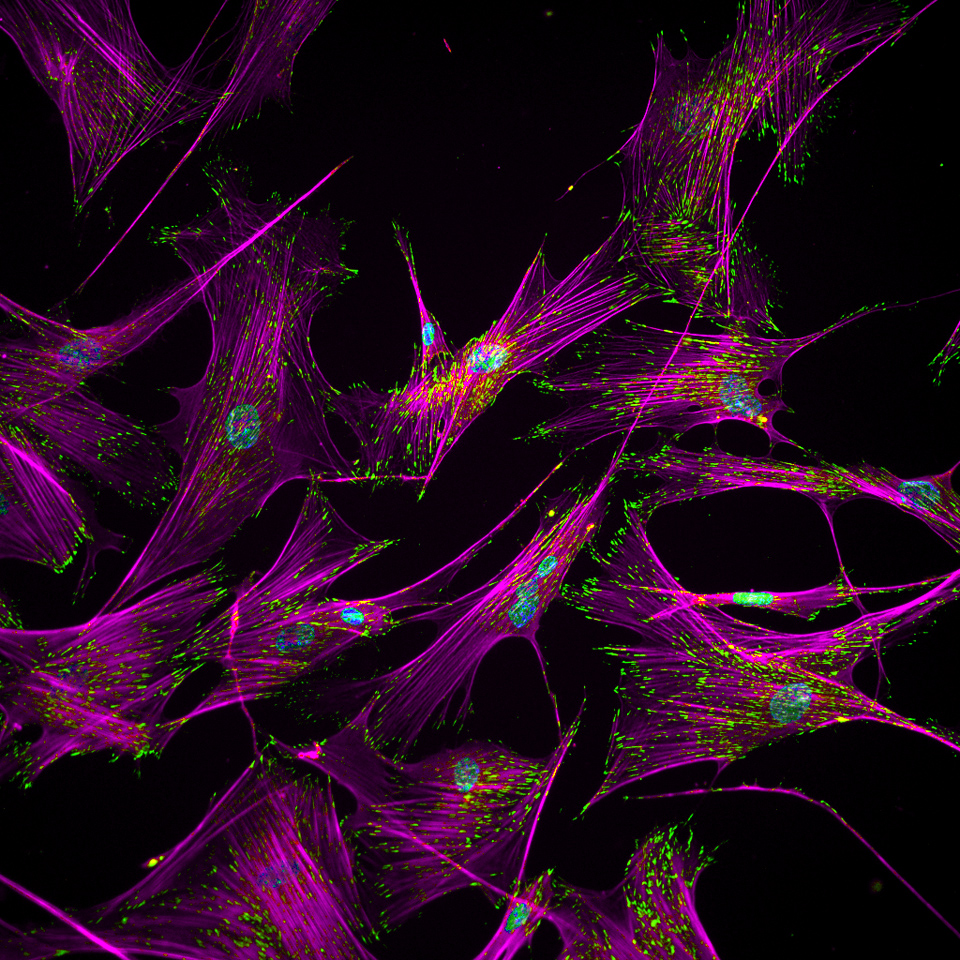
Adult human fibroblast cells with intracellular proteins involved in adhesion of these cells to an extracellular matrix. These fibroblasts are converted to human induced pluripotent stem cells (hiPSCs) through a reprogramming process during which restructuring of the adhesion proteins takes place. Credit: Ankur Singh
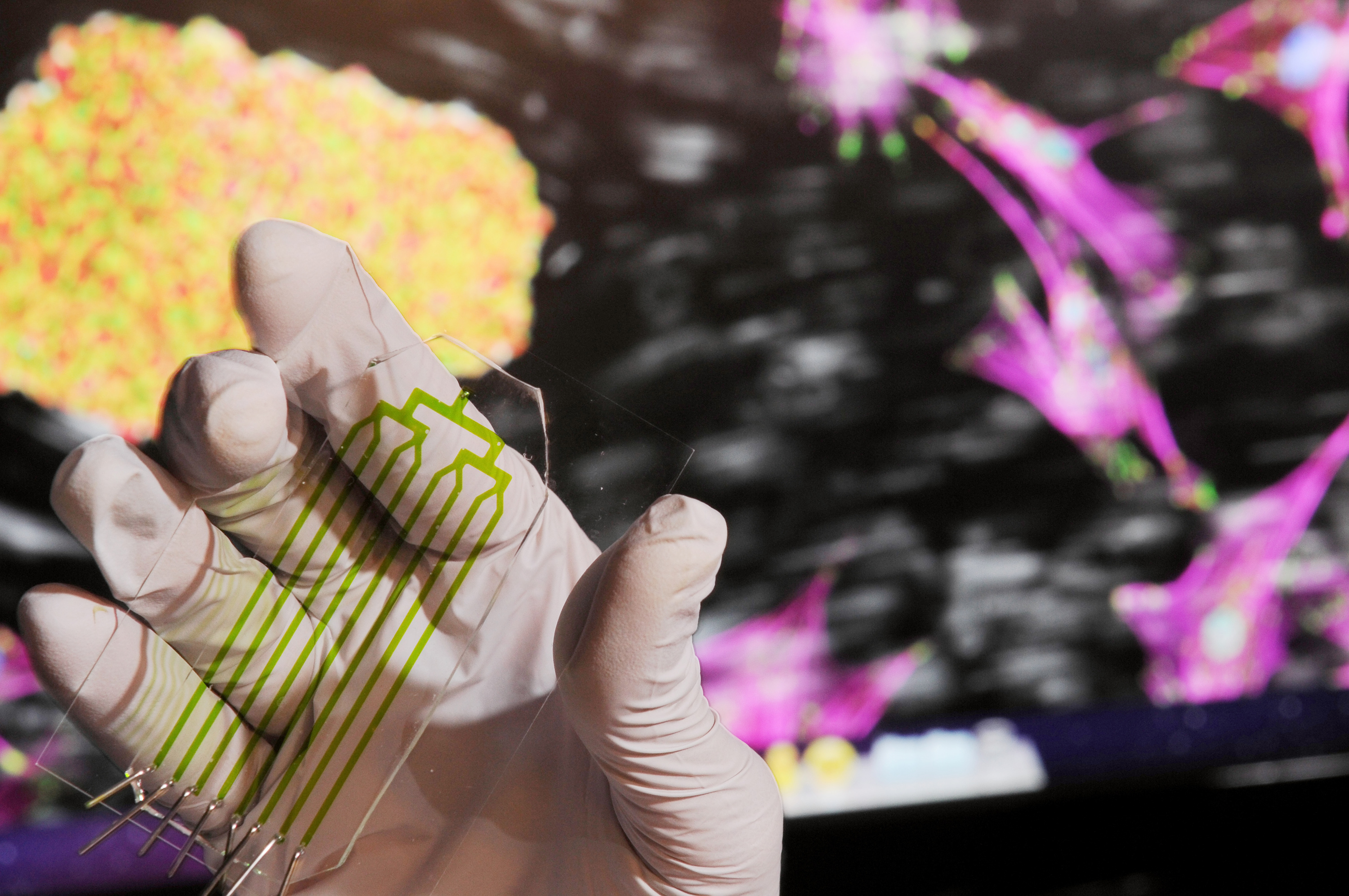
Close-up view of the microfluidic device that exploits the differences in adhesion strength between derived stem cells and contaminating cell types in a heterogeneous culture to selectively isolate cells of interest using fluid shear forces. Credit: Gary Meek
