Only a fraction of infused T cells makes it into targeted tumors
Adoptive cell therapy (ACT), a type of immunotherapy, shows great promise for the treatment of cancer. For an ACT procedure, T lymphocytes (T cells) are extracted from a patient, expanded in a laboratory, and infused back into the patient to enhance their ability to recognize, target, and destroy tumor cells.
Achieving enhanced cancer-fighting capabilities requires an extremely high number of T cells, and only a small fraction of infused T cells makes it into either primary or metastatic lesions. Therefore, predicting which ACT cultures will result in robust tumor homing plays a very important role in ACT efficacy.
Enhance tumor homing and enrich cells exhibiting desired features
Tumor infiltration by CD8+ (cluster of differentiation 8) T cells has been used to improve clinical response with ACT and has resulted in reductions in disease burden and improved survival. Thus, CD8+ T-cell tumor homing and engraftment is considered critical for the success of ACT.
This engineered microfluidic device models the microenvironment of the tumor vasculature ex vivo and incorporates the effects of hemodynamic flow to predict the in vivo homing and engraftment of CD8+ T cells. The method enhances adhesion in flow in vitro, which results in increased tumor homing in vivo by adoptively transferring CD8+ T cells and show improved tumor control in combination with a therapeutic blockade of immune checkpoints.
This new approach has the potential to not only address the delivery limitations of ACT and enhance immunotherapeutic effects, but also yield new insights into subpopulations of CD8+ T cells. It could have wide implications in the field of cancer as well as viral infections.
- Enhances immunotherapeutic effects: Improving the homing capabilities of T cells can increase treatment response and safety while decreasing off-target effects.
- Improves tumor control: Engineered microfluidic devices can identify subsets of cells with enhanced tumor-infiltrating capabilities—a key limitation in the immunotherapeutic effects of solid tumor ACT.
- Optimizes T-cell production: Predicting which expansion protocols yield cells with heightened tumor homing may improve development pipelines by reducing the number of laborious in vivo studies, while also helping cell therapy companies ensure their products reach the desired tissue.
Adoptive cell therapies for:
- Cancer (not limited to pre-clinical models)
- Viral infections
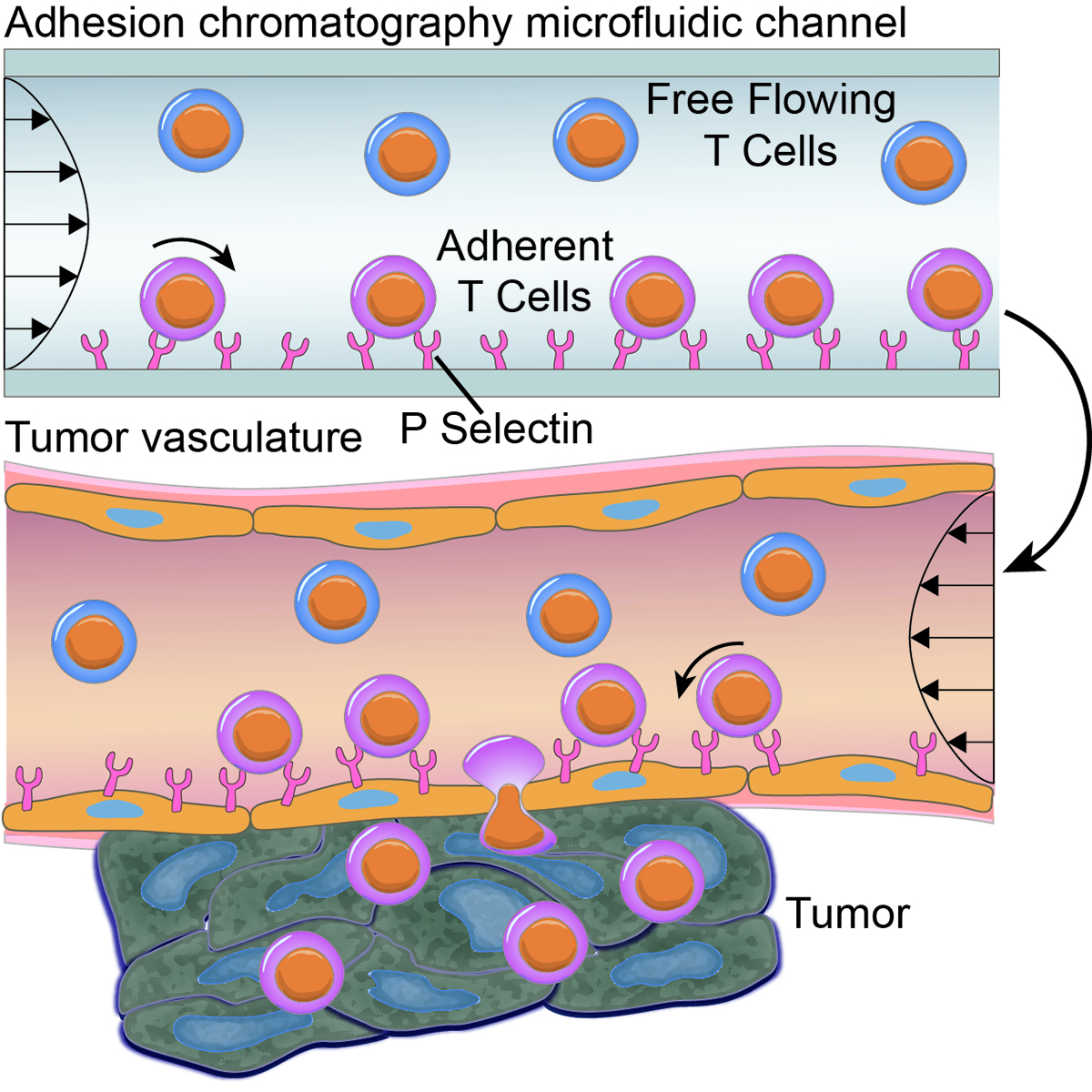
The engineered tumor vasculature-mimicking microfluidic device predicts in vivo tumor homing of CD8+ T cells. The microfluidically enriched CD8+ T cells, for adhesion in fluid-flow in vitro, show superior tumor homing in vivo, remodel the tumor microenvironment, and augment the immunotherapeutic benefit of the immune checkpoint blockade.
