Viral vectors are inherently limited for opsin expression
Opsins are light-responsive ion channels that can be used to control the action potential in cells that exhibit electrical activity, such as neurons and cardiomyocytes. The ability of opsins to control electrical activity in these cells can be measured by microelectrode and microelectrode array (MEA) devices in experiments useful for screening new drugs for cardiac and neurological disorders.
Currently the primary expression vectors for opsins are viral vectors, such as Adeno-Associated Virus (AAV). However, AAV has several limitations, such as slow and permanent expression, immune response induction, possible integration into the genome, and difficulty controlling expression localization—especially when expressing multiple opsins.
mRNA expression vector supports faster, safer transient opsin expression
This innovation is a synthetic mRNA vector used to express opsins in neurons and cardiomyocytes to rapidly induce light-responsive electrophysiological behavior. Unlike viral expression vectors, mRNA will not integrate into the genome, is highly transient in nature, and expresses in 2–24 hours rather than 2–3 weeks. The mRNA vectors provide transient opsin expression with fewer safety risks, while enabling new configurations of optogenetic studies that can include repeated, alternating, or combined dosing with one or multiple opsins. Since opsins can be excitatory or inhibitory, transfection with both types enables alternate opsin expression combinations that can lead to new a paradigm of control networks. Reporter proteins, such as voltage and calcium-sensitive fluorescent proteins, can also be expressed for further characterization of cells.
This technology comprises one or a combination of mRNA encoding opsins, a delivery vehicle, and delivery to neurons or cardiomyocytes plated on a device capable of either optical or electrical excitation and measurement readout. Functional expression of excitatory and inhibitory opsins has been tested using microelectrodes and MEA devices as well as imaging.
Present technologies based on viral technologies do not have the safety profile or expression control offered by an mRNA expression platform. This innovation offers significant benefits for biological research as well as screening of drug compounds for cardiac or neurological disorders.
- Safer: mRNA-based systems have a lower probability of inducing cellular immune responses compared to AAV-based expression systems.
- Faster expression: mRNAs express very quickly and are usually detectable within 2–24 hours rather than the standard 2–3 weeks.
- Better control: The controllable and temporary nature of mRNA delivery offers improved expression regulation based on the transfection amount, especially when using multiple mRNAs that encode different proteins.
- Increased efficiency: MEA measurements show that mRNA vector expression can persist for a longer time—up to 144 hours post-transfection—and have higher light sensitivity.
- Broader applications: Transient expression enables the cells to be re-transfected or tested in some other manner.
- Drug screening for cardiac and neural disorders
- Generation of model systems for testing of new drugs
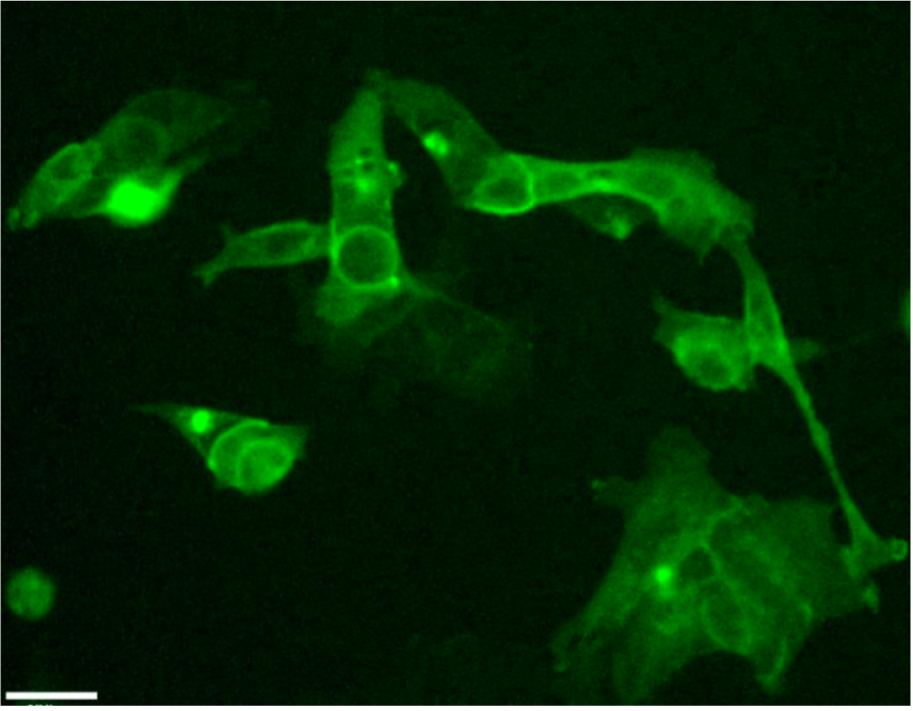
Opsin expression in human embryonic kidney 293 cells (HEK293 cells) 16 hours post-transfection with channelrhodopsin-2 yellow fluorescent protein (YFP) mRNA. Live cells were imaged on a widefield microscope using a YFP filter set and 10x/NA 0.25 objective.
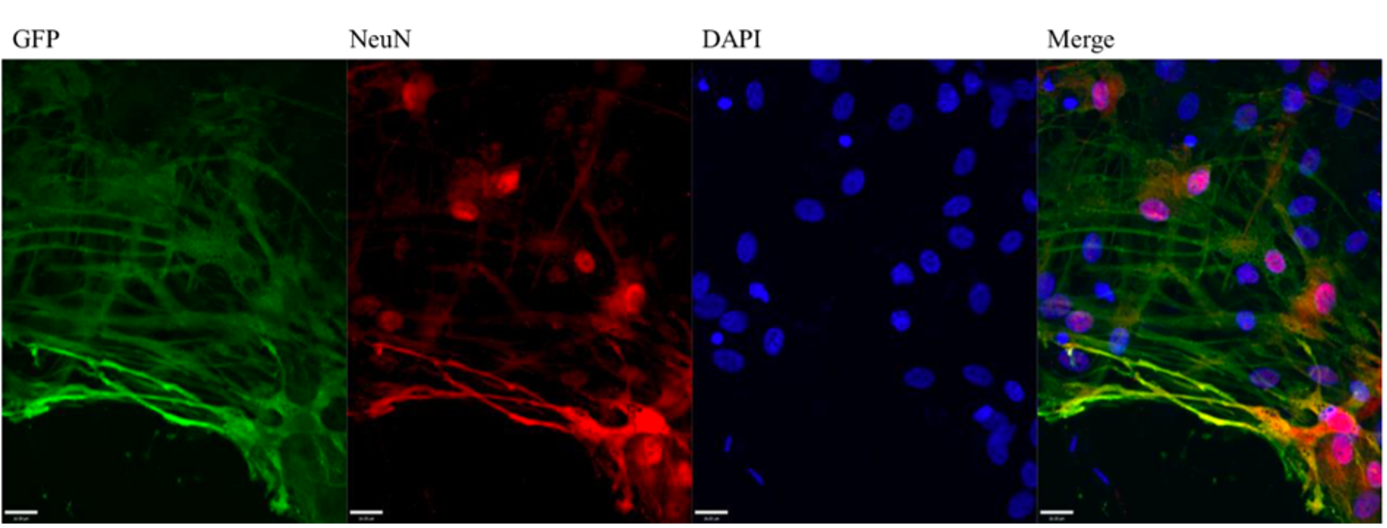
Green fluorescent protein (GFP) expression colocalizes with the neuron-specific nuclear marker NeuN in mixed rat cortical neuronal cultures transfected with GFP mRNA. NeuN (red) marks the nuclei of neurons. GFP is visibly expressed in neurons, though other cell types are visible and may or may not express GFP. Cells were fixed and stained at 24 hours post-transfection and imaged with a 40x 1.2NA objective on an UltraVIEW spinning disk microscope.
UltraVIEW is a registered trademark of PerkinElmer, Inc.
