This innovative Georgia Tech technology overcomes the obstacles of currently available bead-based assays as it can provide a sensitive readout of analyte binding without any intermediate optics. Most bead-based assays in use today depend on expensive optical detection systems, which can limit their widespread use, for example, when needed to detect infectious diseases such as COVID-19. This innovation can be applied to the sensing of any biomarker that can be specifically bound to using a probe, such as proteins, antigens, cells, DNA, RNA, viruses, and bacteria.
The technology uses a bead-based enzyme-linked immunosorbent assay (ELISA) as a first step. This enables analyte binding on antigen-coated microparticles, and the signal is amplified via deposition of silver nanoparticles. Then, the metallized beads flow through a 3D printed micro-aperture, and the change in impedance is captured for each bead as it passes by.
Multiplexing can be achieved by using “impedance-barcoded” beads, where an electronically identifiable property of the bead can be altered that is independent of the electronic signal produced by analyte binding. For example, this could be the shape, porosity, size, conductivity, permittivity, or any combination of the properties.
- Lower cost: Replacing fluorescent markers and readout methods with impedance detection has the potential to significantly decrease the cost of the assay.
- Transportable: Impedance measurements in microfluidics can be made transportable, which can potentially enable point-of-care applications, as electronic elements are more durable than optical elements.
- Practical: Identification of COVID-19 antibodies in COVID-positive serum samples has already been demonstrated.
- Versatile: This technology can be applied to the sensing of any biomarker that can be specifically bound to using a probe, such as proteins, antigens, cells, DNA, RNA, viruses, and bacteria.
- Biological research
- Clinical diagnostics
- Multiplexed assays
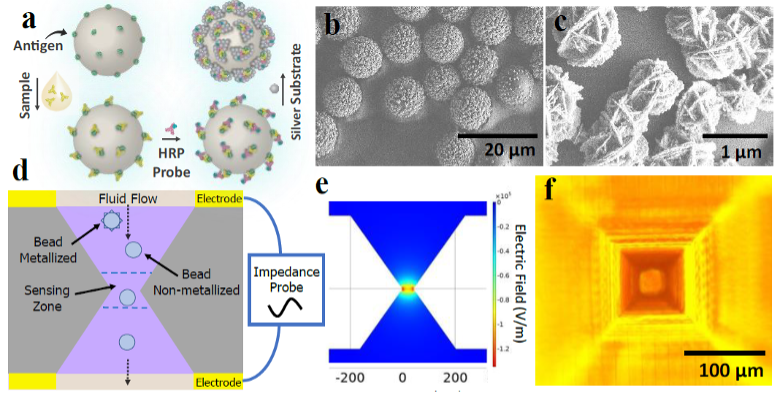
A) Bead-based ELISA protocol; B) Metallized beads; C) Spheroidal silver deposition on bead surface; D) In-flow sensing scheme using micro-aperture; E) Focusing effect of aperture on electric field; F) Micrograph of aperture
