Over 30 million people suffer from valvular heart disease, and close to 200,000 valve replacements are performed each year. Current heart valves are typically permanent mechanical or bioprosthetic devices and have critical limitations. In pediatric patients who grow at rapid rates, these permanent devices quickly become too small, which increases risk of adverse events and necessitates replacements. Although 3D-printed heart valves currently exist and bioresorbable materials have been in use for implants, the two have not been combined.
Patient-specific, bioresorbable heart valves designed to reduce complications and reinterventions while improving patient outcome
Resorbable, patient-specific heart valves offer great flexibility for treating a wider range of conditions and patients than traditionally manufactured heart valves as well as potentially decreasing complications due to poor fit. Made of bioresorbable materials, the valves will eventually be absorbed by the body and replaced by new tissue that will perform the function that the device once served. This is especially important for pediatric patients.
This versatile technique results in devices composed of optimal materials for specific conditions, diseases, and patients. The device can be made from a wide range of polymeric materials to match the mechanical behavior of specific tissues or metallic materials that can also be 3D printed and are bioresorbable. They can also include a combination of both types of materials.
Devices can be personalized to fit the optimal valve geometry, have bulk and surface properties to match the surrounding tissues, and accommodate the delivery mechanism for the specific application (e.g., transcatheter, open heart). They can also be designed to consider patient anatomy, age, condition, or disease as well as speed and cost factors.
Additional customization can include patterned surfaces that help the device better interface with surrounding tissues as well as provide friction to secure it and encourage cell growth. Device materials can be functionalized with proteins, other molecules, drugs, or even cells harvested from the patient to promote cell growth, tissue adhesion, proliferation, vascularization, and more.
- Precise fit: 3D printing enables precise patient-specific designs that offer a better fit, treatment options for more patients, and treatment for a wider range of conditions.
- Reduced risk of complications: Custom-designing resorbable devices specific to patients can potentially decrease risk of complications due to poor fit, reduce the need for reinterventions, and result in overall better patient outcomes.
- Lower treatment costs: Bioresorbable devices can allow growth of new tissues to create a self-sustaining treatment for the long term, potentially eliminating the need for surgical reintervention and lowering overall treatment costs.
- Improved outcomes: To aid in reducing the mismatch between device and tissue and to improve performance and patient outcomes, device materials can be selected for optimal mechanical properties, thermodynamic properties, chemical properties, and surface chemistry and morphology.
- Broader applications: 3D printing of resorbable patient-specific valves provides options for pediatric patients and adults with uncommon diseases and/or anatomical geometries for whom no existing devices can optimally treat.
- Bespoke production process: Devices are custom produced using patient scans and computer-generated models, algorithms to achieve the optimal design based on objective functions (e.g., minimizing thrombosis by designing the valve to reduce pressure gradients), any number of 3D-printing methods or combinations of methods, and numerous material options.
- Faster, simpler prototypes: 3D printing allows for easier prototyping, less time between designing and manufacturing steps, and greater efficiency in manufacturing.
Treat cardiovascular diseases, including but not limited to:
- Regurgitation
- Valve insufficiency
- Bicuspid valve disease
- Valve stenosis
- Congenital heart diseases involving valve atresia, and valvular pathologies due to rheumatic fever
Repair or replace anatomies, including but not limited to:
- Atrioventricular and semilunar valves
- Aortic valves
- Pulmonary valves
- Mitral valves
- Tricuspid valves
Treat patients and conditions for whom no existing optimal treatment devices are available:
- Pediatric patients
- Patients with uncommon diseases and/or anatomical geometries
Various procedures:
- Transcatheter heart valve replacement
- Percutaneous heart valve replacement
- Transfemoral procedures
- Transapical procedures

Heart valves can be fabricated with different geometries via additive manufacturing. This figure contains valves of varying heights, shapes, sizes, etc.
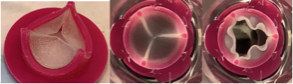
An iteration of the prototype (left). The valve can be seen closing (center) and opening (right) under physiological aortic flow condition via a pulse duplicator.
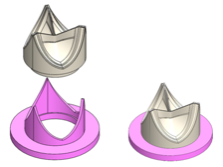
Example of 3D-printed valve assembly where the leaflet portion (beige) can be easily mounted on top of the frame (purple).
