Muscle satellite cells (MuSC) play a central role in muscle regeneration, but their quantity and function decline through physical trauma, aging, and disease, translating to impaired regenerative potential of muscle and may lead to long-term disabilities and reduced quality of life. Current interventions for muscle trauma – primarily muscle flap transfer and surgical suture – do not completely regenerate damaged muscle. Clinical strategies to address injured muscle in aging or disease are needed. Interventions targeting the diaphragm are of particularly critical importance, as damage to the diaphragm can lead to breathing difficulties and potentially death. This is the case with Duchenne Muscular Dystrophy (DMD), and other dystrophic disease. Respiratory care, such as mechanically assisted ventilation, remains palliative. Targeting the diaphragm for MuSC/protein delivery is a promising therapeutic avenue but has been challenging due to the thin dimension of the muscle and its deep-seated anatomic location.
Synthetic hydrogel supports muscle satellite cell survival and proliferation
These engineered hydrogels comprised of synthetic polymers and peptides are designed to enable successful MuSC transplantation to the diaphragm or other dystrophic skeletal muscle tissue, thereby restoring dystrophin – a protein absent in DMD and critical to strengthening and repairing muscle fibers. The hydrogels can be loaded with myogenic agents such as MuSCs and/or pro-myogenic factors to form a bioadhesive matrix with optimal mechanical, cell-adhesive, and protease-degradable properties that support MuSC cell survival, proliferation, and differentiation. The innovation also includes a cell delivery strategy for treating muscle trauma/deterioration by robust engraftment in cases where intramuscular injections may not be applicable. With positive results shown in mouse studies, the hydrogel-based matrix offers a promising treatment for muscle trauma in elderly and dystrophic patients and represents the first work of its kind to enable targeted cell/protein delivery to the diaphragmatic muscle.
- Robust: Includes a biomaterial-mediated cell delivery strategy resulting in strong engraftment that provides an option for treating muscle trauma where injections are contraindicated
- Tunable: Matrix stiffness, mesh size, and degradation rate can be adjusted and cell-adhesive ligands can be incorporated, enabling modulation of a range of critical cellular functions, including survival, proliferation, differentiation, and migration
- Flexible: Enables delivery of a wide range of therapeutics, including cells, proteins, and small molecule drugs
- Non-sutured delivery: Uses thiol-maleimide chemistry to covalently adhere cells to the diaphragm (or other muscle) surface without puncturing the muscle, unlike prior (unproven) attempts that required surgically stitching a decellularized scaffold to the diaphragm
- Tested: Demonstrated significantly improved in vivo cell survival, proliferation, and engraftment in non-irradiated and immunocompetent muscles of aged and dystrophic mice compared with collagen gels and cells-only controls
- Adaptable: Provides the opportunity to treat previously unaddressed diaphragmatic deterioration and resulting respiratory failure and may serve as an important foundation for further bioengineering research and ultimately patient care
The synthetic hydrogel for satellite cell delivery platform is applicable to further research and bioengineering to develop treatments for muscle trauma and deterioration as well as dystrophic disease, including:
- Duchenne Muscular Dystrophy
- Other muscular dystrophies (Becker muscular dystrophy, Congenital muscular dystrophies, Limb-girdle muscular dystrophies, Myotonic dystrophy, and others)
- Ventilator-Induced Diaphragm Dysfunction
- Craniofacial and limb muscle trauma
- Post-operative wound treatment in elderly and dystrophic patients
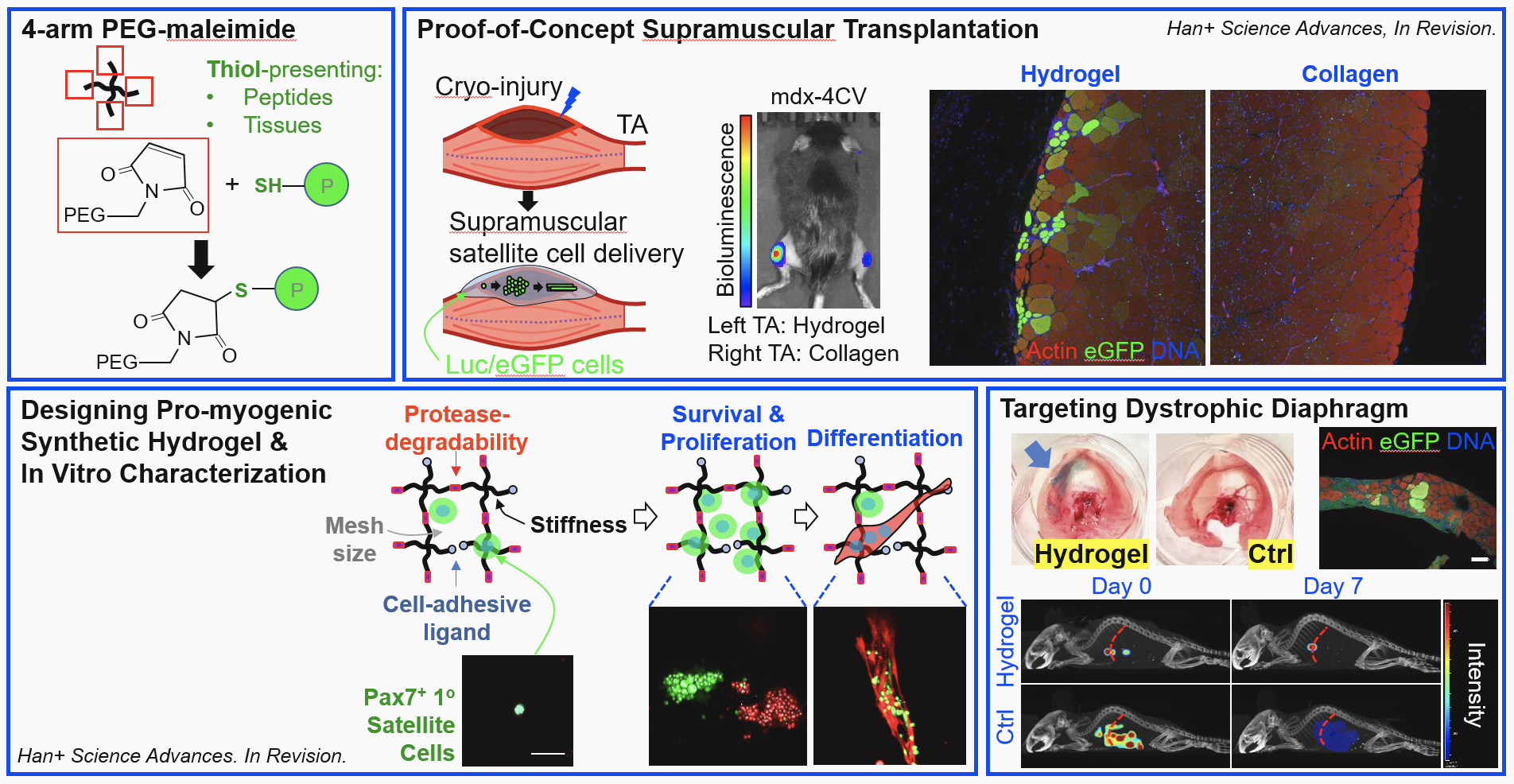
The synthetic hydrogel for satellite cell delivery uses thiol-maleimide chemistry (upper left) to covalently adhere cells to the diaphragm or other muscle. A proof-of-concept study in mice (upper right) showed promising results compared with collagen gels. The hydrogel platform is highly tunable (bottom left) in terms of mesh size, stiffness, and other factors that impact MuSC survival, proliferation, and differentiation. A critical use case is the diaphragm (lower right), particularly in dystrophic patients.
