Current artificial heart valve replacement treatment options lack safety, specificity, and longevity
There is a need for artificial heart valves that provide a safer, more durable, and less expensive treatment option for patients, especially pediatric patients, with congenital and other heart defects (e.g., aortic and pulmonary stenosis). Existing prostheses for pediatric and adult patients may lead to adverse events, including calcification, organ rejection, endocarditis, and stenosis. Particularly for pediatric patients, the use of smaller adult tissue prostheses may require lifelong anticoagulation and risks limited longevity due to increasing patient-prosthesis mismatch. Materials currently used in manufacturing artificial valves are costly and often unavailable, creating another impediment in the scalable production of current medical devices. A device that offers biocompatibility and customization with readily available, inexpensive materials would greatly decrease the incidence of post-surgical complications while improving durability and affordability.
Innovative process creates biocompatible, patient-specific heart valve replacement device
This new process creates a biocompatible, antithrombotic, calcification-resistant valved conduit for heart valve replacement by incorporating hyaluronic acid (HA) into linear low-density polyethylene (LLDPE). With pre-surgical computational planning, each device can be customized to precisely fit each patient’s anatomy, potentially providing easy surgical placement and improved hemodynamics. This innovation is manufactured via thermoforming, incorporating polyethylene (PE) rings at the ends of the conduit for suturing the device to native tissue and allowing the addition of a sinus when necessary. The unique nature of the HA/LLDPE material may reduce the incidence of many known adverse events and may offer a longer lasting, more effective, and less expensive solution.
- Biocompatible: This technology may reduce risk factors and incidence of post-surgical adverse events.
- Customizable: Pre-surgical computations allow patient-specific device creation, with optional sinus addition during manufacturing.
- Safer: Patient-specific design potentially allows easy surgical placement and improved post-surgical prognosis.
- Durable: A biocompatible, customized device may lead to long-term success rates in the absence of risk factors.
- Less expensive: Materials are readily available and relatively inexpensive compared to those in current use.
- Tested: This technique has demonstrated increased blood compatibility, reduced calcification, and prevented immune rejection of PE heart valve leaflets in vitro and in vivo.
This new method and device have applications in cardiovascular surgeries aimed to treat:
- Congenital heart defects
- Other heart defects, such as aortic and pulmonary stenosis
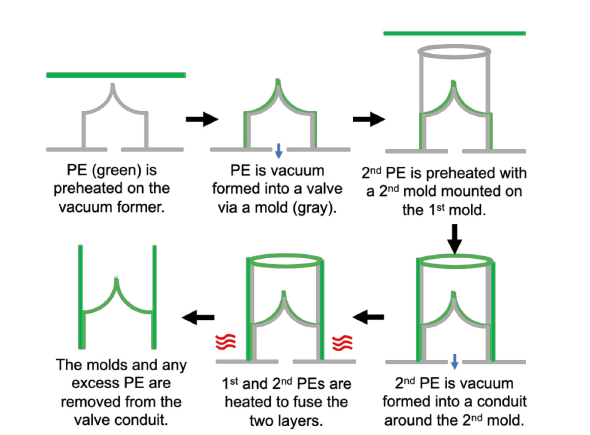
Schematic of the thermoforming process to form the valved conduit
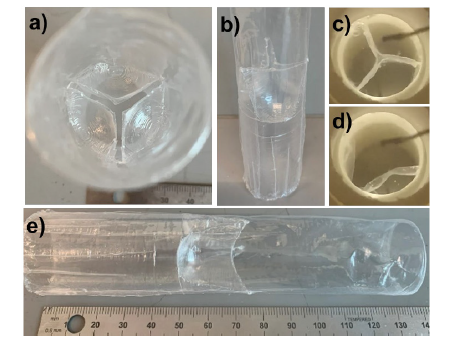
A functioning prototype of the valved conduit. Face (a) and side view pictures of the clear plastic LLDPE valved conduit (b and e). Still images of the conduit under pulsatile flow during peak diastole (c) and systole (d).
