To increase the accessibility of life-saving medicines and vaccines, researchers have developed Smart Porous Hydrogels (SMH) to serve as polymer-networked vessels for various biologics and pharmaceuticals. The SMH start out as dry units designed to absorb the intended therapeutic or vaccine suspended in water when the two are introduced.
The value of the SMH lies in its absorbency and porousness. Its absorbency is designed to take in a large volume of water that is rich with the intended active components. Meanwhile the network of polymers with a well-chosen biological stabilizer creates a mesh with a specified pore size for a greater uptake of the suspended biologic or pharmaceutical while rejecting a significant number of impurities (e.g., >90% of E. coli, ~80% of S. epidermidis). This selectivity should allow for a considerably high recovery rate when stimuli are applied to the SMH and the active components are freed.
When the level of impurities is low and the recovery rate after stimuli is high, the biodegradation of the biologic or pharmaceutical liquid decreases and should allow for a longer shelf life than the current standard process, even when there is a failure in the cold supply chain. Additionally, by immobilizing the medicine in the SMH, there is less risk that self-degradation would occur in transportation and storage. With the possibility of decreased biodegradation and self-degradation simultaneously, this proposed process may extend the viability of these pharmaceuticals and biologics, resulting in less waste for pharmacies and greater accessibility for patients.
- Preservation: With the ability to adjust the pore size of the SMH, researchers may be able to increase the rejection rate of bacteria, thereby protecting the medicine by slowing down biodegradation.
- Extended use: Preserving the potency of the transported and stored biologic or pharmaceutical should allow for an improved shelf-life (regardless of the cold supply chain) and an increase in the supply accessible to patients.
- Lower cost: Using Georgia Tech’s SMH for the storage and handling of biologics or pharmaceuticals should minimize the dependence on a cold supply chain, which utilizes freezers that cost between $10,000-$15,000.
This technology is expected to assist the supply of pharmaceuticals and biologicals to remote locations, resource-limited regions, and/or developing nations:
- Vaccine deployment
- Insulin supply chain
- Ear, nose, and throat (ENT) medications
- Antibiotic packaging and supply
Many biologics and pharmaceuticals rely on cold supply chain for transport and storage until they are administered or supplied to the patient, including but not limited to vaccines, insulin, ENT drops, and some antibiotics. The cold supply chain has many variables that may cause loss of supply, decreased potency of the medicine, or add significant financial burden to both the pharmacy and patient. The importance of maintaining the cold supply chain integrity has been brought to the forefront because of the practicality of bringing the COVID-19 vaccine to rural and developing regions both in the United States and around the world.
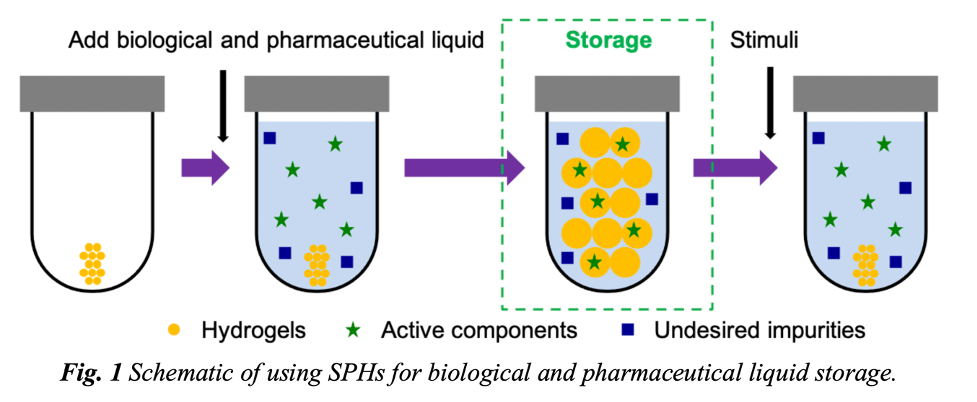
Schematic of using Smart Porous Hydrogels (SPHs) for biological and pharmaceutical liquid storage.
