This density-driven cell seeding method promotes cell attachment to the underside of a flat surface or membrane without the need for inversion by enabling cells to float. The technology achieves flotation by modulating the density of the cell suspension medium so that the cells are less dense than the medium itself. Georgia Tech’s innovation has been demonstrated in a cell assay using a 96-well model of the small airway-vascular barrier with serum-free, glucocorticoid-free air-liquid differentiation. The polarized epithelial-endothelial co-culture exhibits mature barrier function, appropriate intercellular junction staining, and epithelial-to-endothelial transmission of inflammatory stimuli such as Poly(I:C). Notably, exposure to influenza A virus [PR8 strain] and human beta-coronavirus [OC43 strain] has been shown to initiate a dose-dependent inflammatory response that propagates from the epithelium to endothelium.
While Georgia Tech’s model focuses on the air-blood barrier, this underside seeding method is generalizable to preparation of various co-culture tissue barrier models for scalable physiologic screening. It can be applied to other organ barriers as well, including the gut, skin, oral mucosa, and other organ systems of interest for drug discovery.
- Convenient: Eliminates the need for inversion procedures, which tend to be tedious, difficult to automate, and prone to failure—especially for 96-well inserts due to their limited culture area
- Generalizable: Developed for screenings that focus on the air-blood barrier, but can also be applied to gut, skin, oral mucosa, and other organ systems
- Scalable: Employs a simple method that is extensible to automated processes for high-throughput drug screenings and pharmacological tests
- Economical: Uses readily available reagents and liquid-handling equipment and does not require reconfiguring of robotic systems to invert large quantities of plates, making for a low-cost solution
- Effective: Demonstrates a high success rate of seeding using a 96-well plate assay with an average of 93 out of 96 wells meeting quality standards in laboratory testing
- Drug discovery
- High-throughput cell-based screening
- In-house co-culture modeling
- Expanded use of existing automated culture handling systems
- Fabrication of novel pulmonary barrier testing platforms
- Generation of bilayer co-culture models for screenings
High-throughput tissue barrier models can yield critical insights on how barrier function responds to therapeutics, pathogens, and toxins. However, such models often emphasize multiplexing capability at the expense of physiologic relevance. In particular, the airway-vascular barrier is typically modeled with epithelial cell monoculture, but this neglects the substantial contribution of endothelial cell feedback in the coordination of barrier function. An obstacle to establishing high-throughput co-culture models relevant to the epithelium-endothelium interface is the requirement for underside cell seeding, which is difficult to miniaturize and automate. Georgia Tech’s method enables completely automated inverted seeding, eliminating a manufacturing bottleneck that has prevented cell-based high throughput screening from advancing into bilayer co-culture.
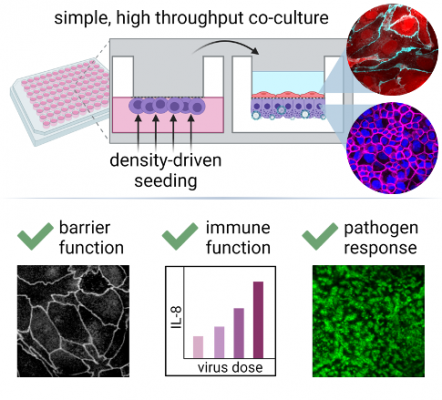
A high throughput bilayer coculture model of the distal air–blood barrier is developed using a novel underside seeding method that requires no filter inversion. The cocultured small airway epithelial–endothelial barrier demonstrates physiologic responses to pathogen mimics and live virus exposure, including barrier loss, cytokine production, and von Willebrand Factor release.
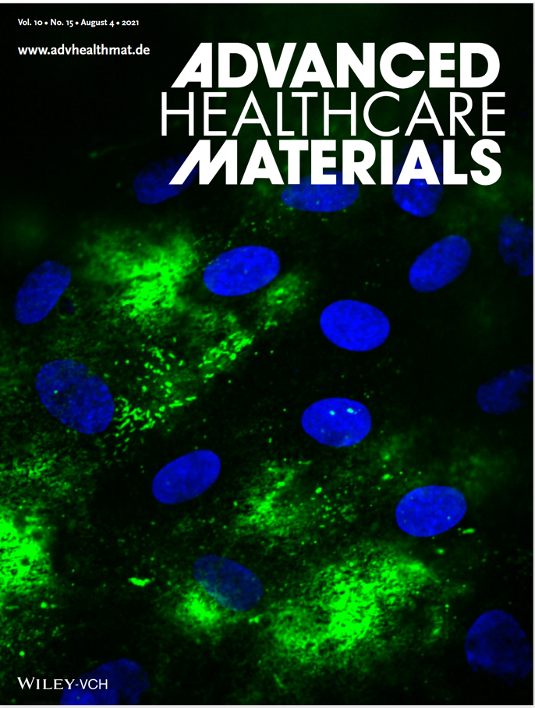
The confocal image (60x) here shows a higher resolution of work in Advanced Healthcare Materials article number 2100879 by Shuichi Takayama and colleagues. Epithelial cells are stimulated with poly(I:C) resulting in endothelial release of von Willebrand Factor (green). This demonstrates that the culture model allows propagation of inflammatory signals from epithelium to endothelium.
