The most common serological testing methods utilize either lateral flow assays (LFAs) or ELISAs to detect antibodies induced by past infections. The low cost and convenience of LFAs, which wick samples into a porous substrate via capillary action and visually report target antibodies as colored bands, make it the preferred assay format for POC testing. However, the method’s convenience comes at the expense of quantitative results. The binary colorimetric output of LFAs cannot report the antibody titer—an important parameter that is closely correlated with the degree of immunoreactivity against a pathogen or a transplant.
ELISA testing is the gold standard for detecting target molecules in bioassays, including the serological assays that quantitatively measure specific antibody titers. However, determining antibody titers using ELISA is labor intensive and involves multiple steps that must be performed by trained staff in clinical laboratories. Because the sample must be serially diluted and distributed over an array of wells, the overall complexity of the process makes it challenging to adapt ELISA assays for POC use.
Paper-based dipstick provides multi-well ELISA for POC testing
This new paper-imprinted LFA automatically performs a multi-well ELISA assay for POC titer measurements. Eliminating the numerous, time-consuming pipetting steps typically required, this device serially dilutes the target analyte and distributes it to individually isolated wells to create a concentration gradient. The assay measures titer by gradually immuno-depleting the target analyte from a flowing sample. It also automatically and programmatically performs the delivery of reagents based on preset incubation times required to execute an optimal ELISA protocol. This automation is achieved with a lateral flow device equipped with an imprinted flow controller that is programmed with delaminating timer gates.
This technique has the potential to transform a variety of ELISA-based tests exclusively performed at clinical laboratories into single-use, disposable dipstick tests to be used at the point of care or home.
- Simplifies ELISA testing: This technology eliminates the need for centralized laboratories staffed with trained personnel who perform multiple steps in a specific sequence to execute the assay workflow.
- Enables ELISA-based POC testing: A variety of ELISA-based tests currently performed exclusively in clinical laboratories are potentially transformed into single-use, disposable dipstick tests to be used at the point of care or at home.
- Flexible and proven: The assay can be adapted to multiple conditions. Assay measurements have been demonstrated in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody titers (IgM/IgG antibodies to nucleocapsid and spike protein) and troponin I (a cardiac biomarker).
- Designed for point-of-care or home testing for the qualitative and quantitative study of immune responses in order to:
- Determine precise rates of infection
- Identify potential convalescent donors for serum/plasma therapeutics
- Evaluate vaccine efficacy
- Assess protective immunity from reinfection
- Offers potential for sensitive and specific detection of pathogens, hormones, drugs, or metabolites.
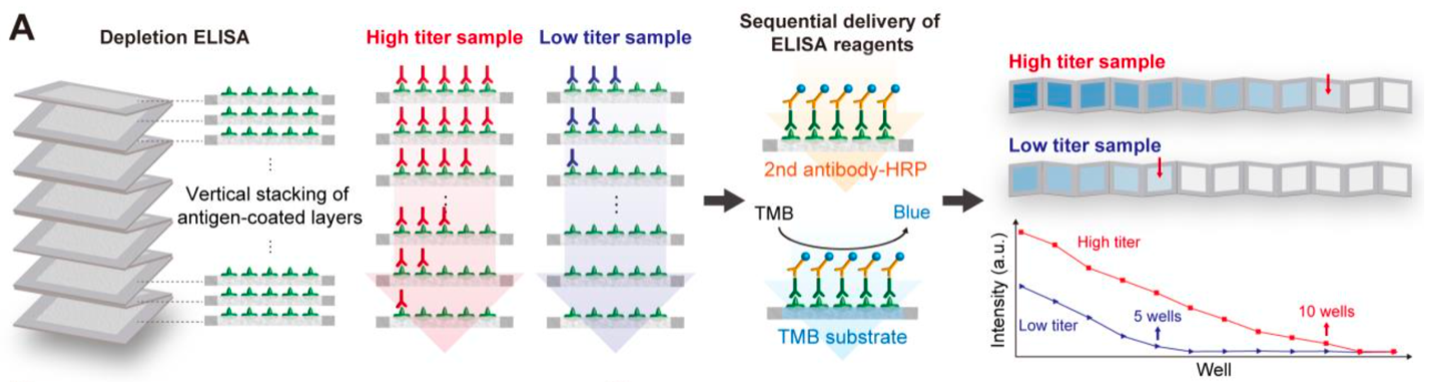
An illustration of Georgia Tech’s depletion ELISA using vertically stacked paper layers with discrete wells demarcated by water-insoluble ink. Each layer was functionalized with a recognition element (e.g., antigen). The target analyte (e.g., antibody) applied on the top layer is gradually depleted by immunocapture, effectively diluting the analyte during flow to create a concentration gradient. The presence of immunocaptured analyte on layers is transduced into an optical signal, and the antibody titer can be determined by counting the number of colored wells.
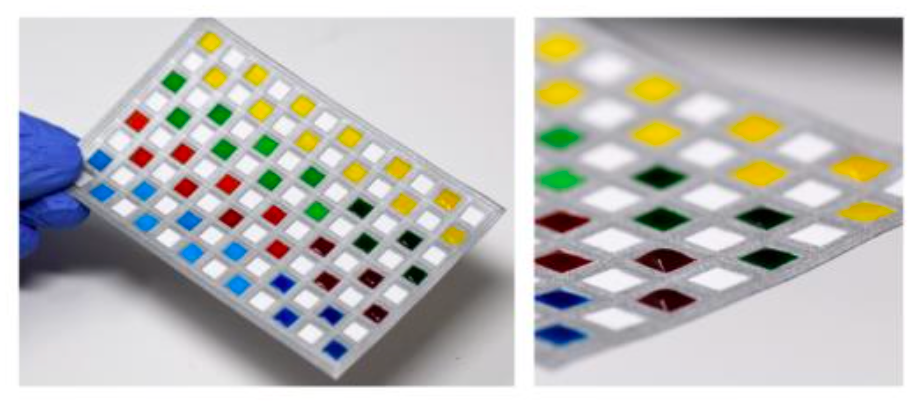
Photographs showing the paper-based 96-well sheet. Applying four dye solutions to individual wells confirmed complete isolation between wells with no leakage observed between adjacent wells.
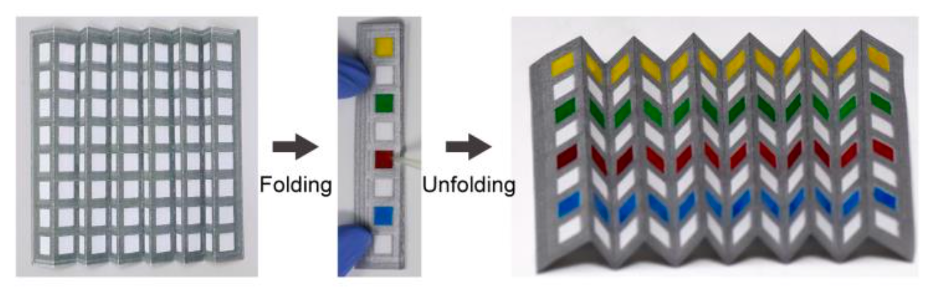
Photographs showing (left) 96-well plate unfolded without application of dyed solutions, (middle) after dyed solutions were introduced to every other stacked well on the folded device, and (right) after the 96-well plate was unfolded following application of dyed solutions. The images illustrate successful isolation during vertical transit through the folded well-sheet.
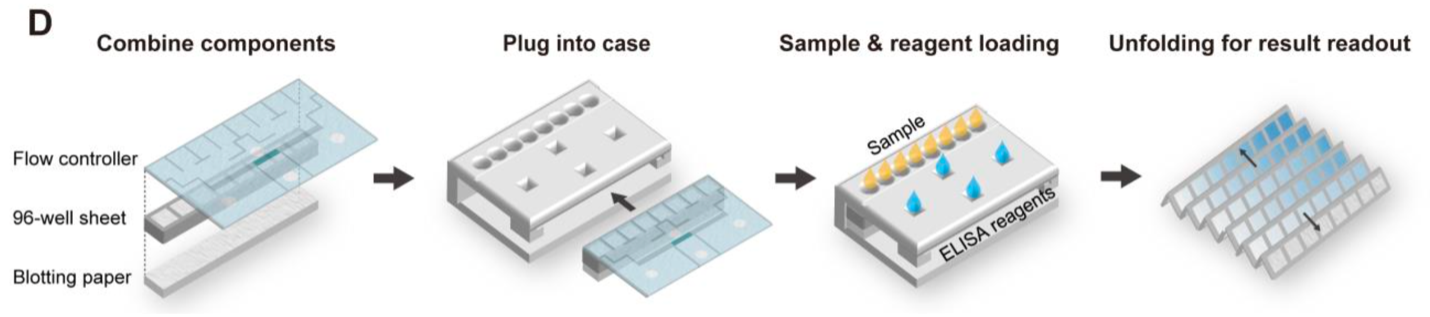
A schematic showing the procedure to operate the assay. Sample and ELISA reagents were simultaneously introduced from different inlets on the device case enclosing all components. Assay results were read out by unfolding the 96-well sheet.
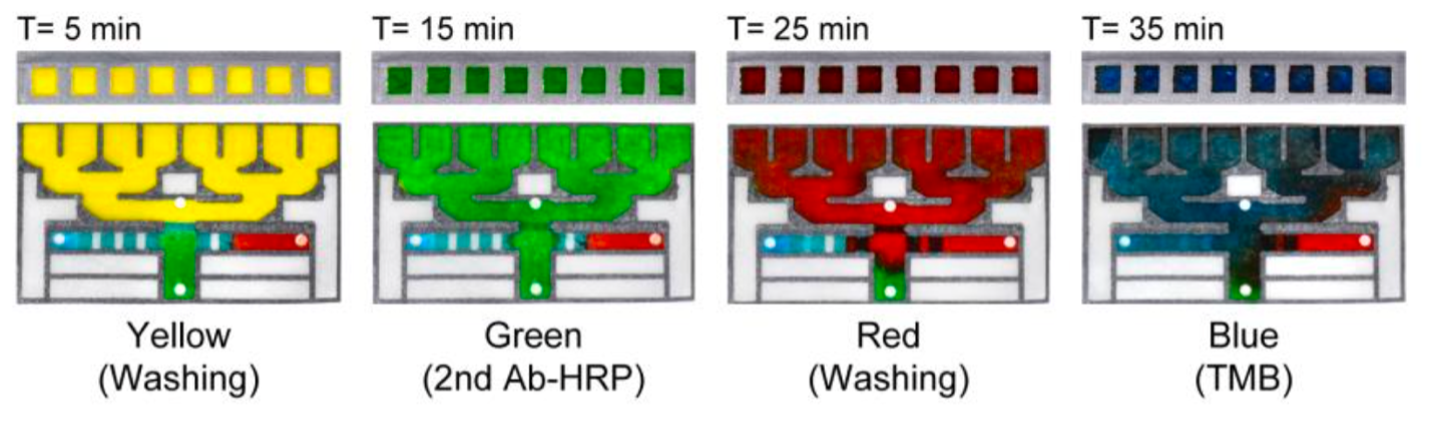
Time-lapse images showing the automated routing of ELISA reagents by the programmed flow controller. The images confirmed as-programmed sequential delivery of washing buffer (yellow), HRP-conjugated secondary antibody (green), another washing buffer (red), and TMB substrate (blue) with preset incubation times required to execute an optimal ELISA protocol.
