This novel fabrication technique establishes a high-throughput phenotypic assay for fibrosis, the development of fibrous connective tissue as a reparative response to injury or damage. Coordinated fibrinolysis and collagen deposition are critical components of wound healing, and there is a need for in vitro assays to evaluate the combined effects of these processes on cell-mediated fibrin remodeling.
Georgia Tech’s assay leverages printed microscale fibroblast-laden fibrin matrices and time-lapse microscopy with a customized label-free image processing approach that enables high-throughput evaluation. It can be used to evaluate anti-fibrotic compounds and their effects on the in vitro matrix remodeling process.
This technique utilizes aqueous two-phase systems (ATPS) to control thrombin-mediated enzymatic crosslinking of fibrin during construction of cell-laden scaffolds. The approach offers a variety of potential applications in personalized medicine, diagnostics, and drug discovery. Additionally, it can be adapted to fabricate arrays of single fibroblast fibrin gels to analyze fibrin remodeling on the single-cell level.
- Powerful: Provides high-throughput image-based analysis of fibrotic processes as well as fibrinolysis
- Convenient: Combines label-free readouts for fibrin degradation, collagen synthesis, and cell contraction to evaluate fibrotic tissue remodeling
- Enabling: Opens new opportunities for understanding fibrosis pathogenesis and for evaluating anti-fibrotic therapeutics
- Identification of novel anti-fibrosis therapies
- Therapeutic strategies for personalized medicine using patient-specific tissue
- Diagnostic tools for evaluating fibrosis biomarkers using patient serum samples
- Quality control for cell-based therapies
Degradation of the provisional fibrin matrix is a key process in wound healing. Following tissue damage, fibrin serves as a temporary scaffold that enables fibroblasts to migrate to the injury site for remodeling of the extracellular matrix (ECM), a dynamic network of collagens, elastic fibers, and proteins that give structure to cells and tissues. Accelerated fibrin degradation can delay healing by hindering cells’ ability to migrate into the wound, while suppressed fibrin degradation can promote fibrotic scarring by contributing to excessive collagen accumulation. Properly regulated fibrinolysis is crucial to wound resolution; however, most currently available phenotypic assays are unable to evaluate cell-mediated proteases and inhibitors as well as biomechanical cellular processes that combine to influence fibrinolysis.
Georgia Tech’s novel technique provides a high-throughput phenotypic assay to evaluate cell-mediated fibrin remodeling. Further, the ability to conveniently evaluate the cumulative effects of fibrinolysis and collagen deposition using small numbers of primary human fibroblasts opens new opportunities for understanding fibrosis pathogenesis and for evaluating anti-fibrotic therapeutics.
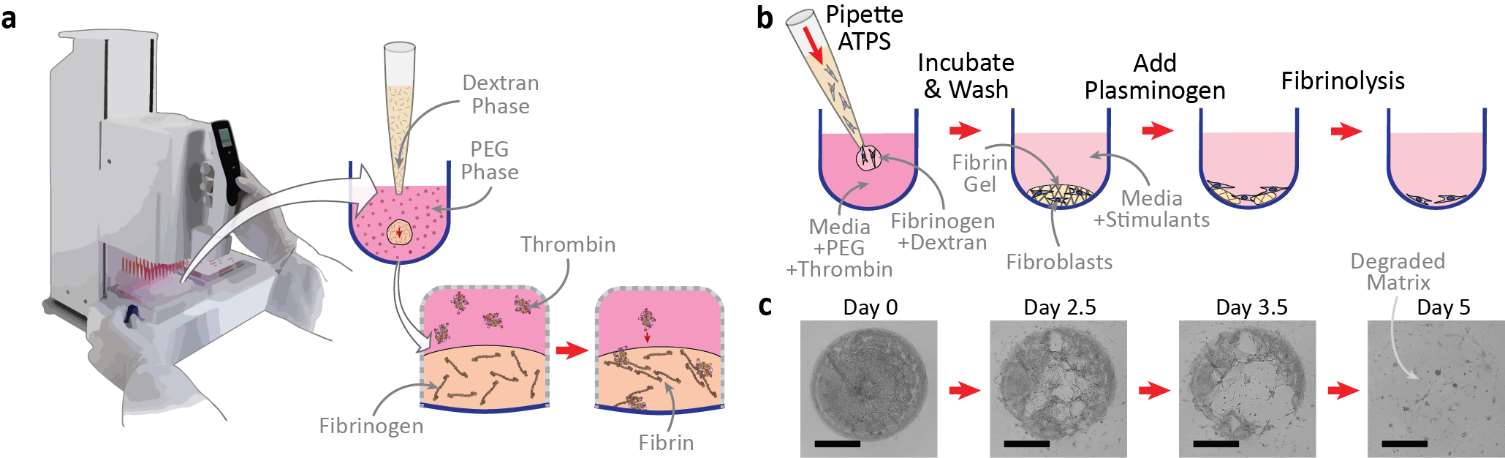
ATPS fibrin printing and cell-mediated degradation: (a) Enzymatic control enabled by ATPS printing of fibrin scaffolds, (b) ATPS generation of microscale fibrin droplets and subsequent fibrinolysis, (c) Characteristic brightfield microscope images (taken at 4x magnification) illustrating assay progression
