Orthopedic device infections, most commonly involving Staphylococcus aureus, often lead to implant failure and subsequent removal. Successful treatments are limited because of the inability of antibiotics to mitigate bacterial biofilms, whose matrix provides protection from the host immune system and acts as a diffusion barrier for antibiotics, thereby increasing resistance. Lysostaphin is a bacteriolytic enzyme with high anti-staphylococcal activity that reduces the biofilm and promotes lysis. The lack of an effective delivery method for lysostaphin, however, has limited its use. An injectable method for treating infections with lysostaphin while promoting bone regeneration is needed.
Hydrogel technology improves infection prevention and treatment while enabling better bone regeneration
This poly (ethylene glycol) (PEG) hydrogel technology provides a bifunctional material that both promotes repair and prevents failure due to infection. Encapsulating lysostaphin in an injectable hydrogel formulation enables efficient, localized delivery that conforms and adheres to the fracture and surrounding tissue. Enzyme stability and activity is maintained, and anti-biofilm activity is enhanced. Studies indicate that the technology has the potential to effectively eliminate orthopaedic S. aureus infections while simultaneously supporting fracture repair.
- Efficient local delivery: Injectable (or surgically applied) hydrogel formulation conforms and adheres to the fracture and surrounding tissue, ensuring efficient, local delivery of lysostaphin
- Higher doses: Localized treatments enable higher relative doses to be given at the site of infection
- Potentially fewer treatments: Enhanced stability from the hydrogel carrier may reduce the number of overall doses needed
- Greater control: Tunable nature of the material provides control over the amount and time over which lysostaphin is delivered
- Faster results: Out-performed prophylactic antibiotic and soluble lysostaphin therapy after 7 days in a mouse model of femur fracture, demonstrating clearance of bacteria and associated inflammation
- Potentially higher success rates: Infected fractures treated with lysostaphin-delivering hydrogels fully healed by 5 weeks with equivalent bone formation and mechanical properties to uninfected fractures, while fractures treated without the hydrogel carrier were equivalent to untreated, non-healing infections
The applications for this technology involve the treatment of Staphylococcal infections, including antibiotic resistant strains.
- Treating S. aureus and S. epidermidis infections, including hard-to-treat infections that may include a bacterial biofilm
- Increasing orthopedic device implant safety success and promoting bone regeneration
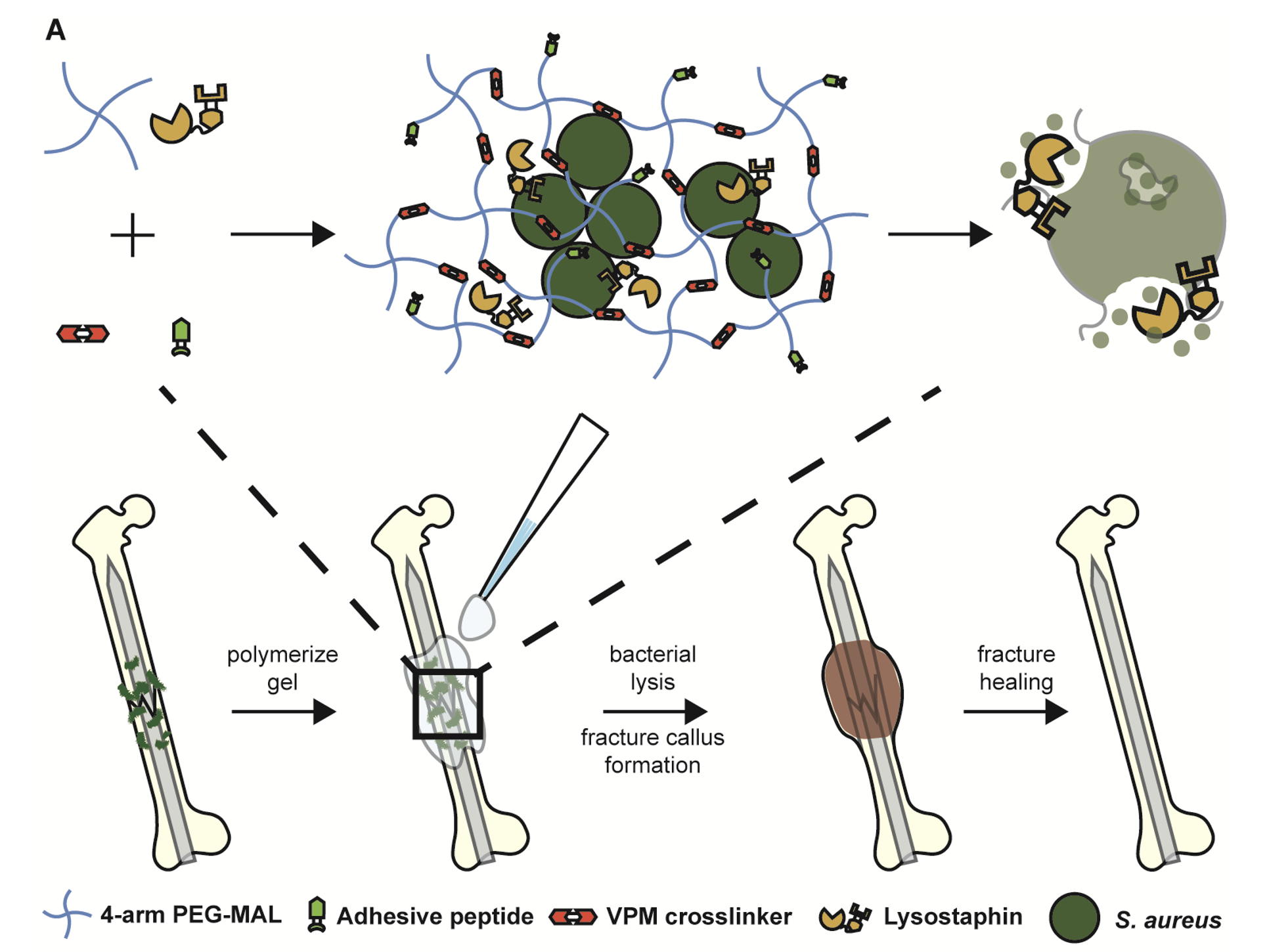
Figure 1. Schematic diagram of lysostaphin encapsulation within protease degradable PEG-MAL hydrogel and subsequent application to infected femurs, which leads to fracture callus formation and healing.
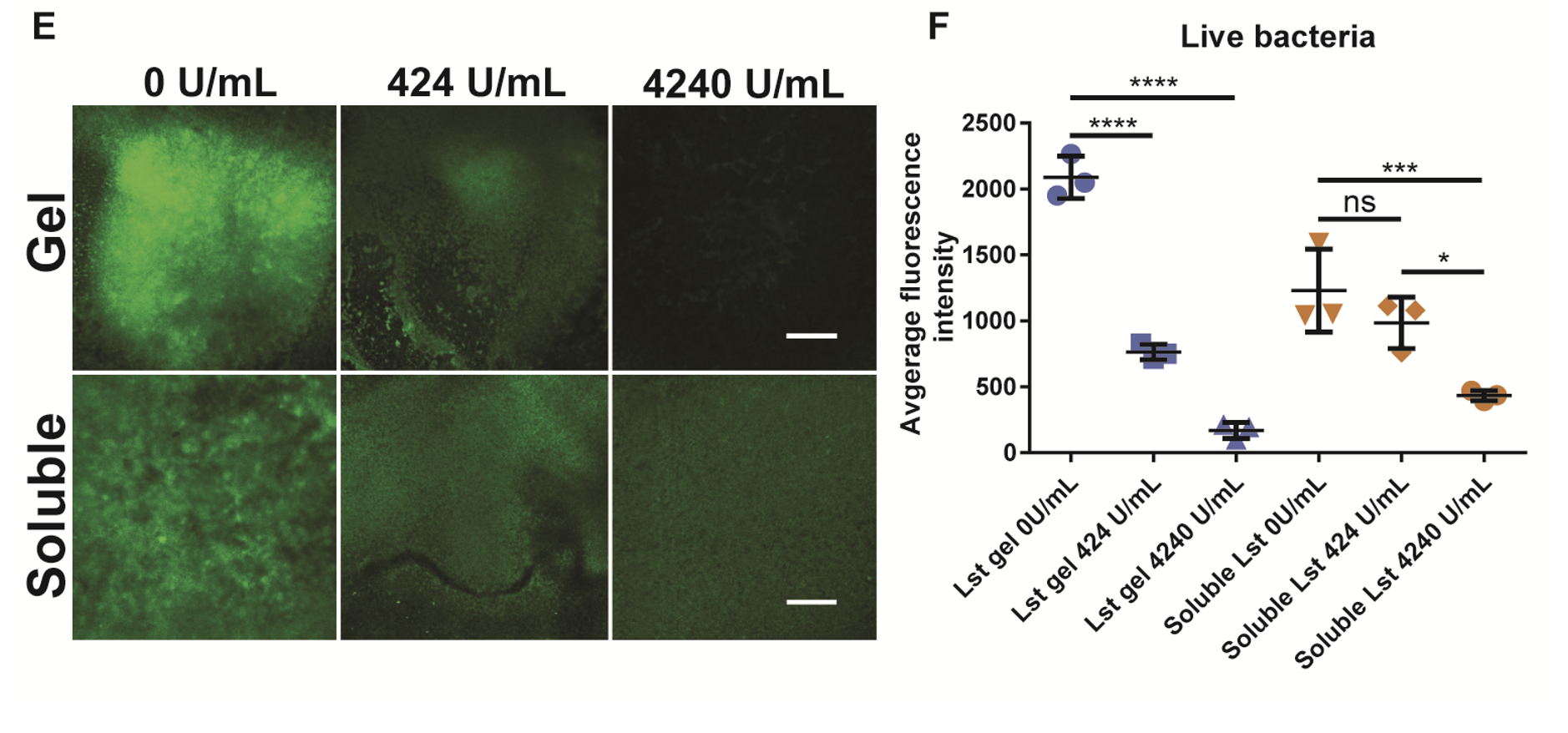
Figure 2. Biofilms were grown for 24 hours and then treated overnight with a hydrogel or soluble enzyme. (E) Shows images and (F) shows the quantification of average image intensity of live bacteria after treatment.
