Existing non-biodegradable ACL replacement prostheses suffer from mechanical failure and bio-incompatibility.
The standard practice for repair of torn anterior cruciate ligaments (ACLs) is ligament reconstruction via autografted tissue. This procedure, however, carries certain risks and challenges, such as donor-site morbidity, initial low strength, high probability of post-operative rupture, and a long and painful recovery period. Artificial ligaments offer substantial improvements to existing therapies; however, attempts to create non-biodegradable ACL replacement prostheses have had unsuccessful long-term results in vivo due to mechanical failure or bio-incompatibility.
Non-biodegradable, biocompatible device mimics natural ligaments to significantly reduce patient risk.
This hydrogel-based device is a non-biodegradable, biocompatible technology for ligament or tendon repair. It aims to provide the required tensile strength and strain to reproduce the mechanical properties of natural ligaments and tendons by possessing a water content and molecular structure closely related to living tissue. The device has two parts with different properties: the flexible median part (or body) that mimics the natural ligament or tendon and the end part that anchors to the bone with different tensile stiffness. The device is designed to be placed in vivo using minimally invasive techniques without requiring removal of healthy tendons or ligaments from the patient. While the artificial ligament or tendon is generally elongated with the anchoring system on at least one end for attachment to the bone, it may take any form necessary to repair the natural section.
- Biocompatible: Hydrogels have a water content and molecular structure analogous to living tissue, providing a non-toxic reconstruction material that decreases immunologic responses.
- Safer: This device is designed to be deployed using minimally invasive techniques.
- Durable: The non-biodegradable nature of this invention confers a high likelihood of durability in vivo.
This device can be used in multiple orthopedic conditions, including:
- ACL replacement
- Replacement of other ligaments and tendons (e.g., wrist ligaments, rotator cuff, etc.)
- Manufacture of synthetic extra-cellular matrix
- Drug delivery – At least one compound may be imbedded in the hydrogel structure to aid in successful reconstruction. Examples include growth factors, anti-inflammatories and mineral fillers.
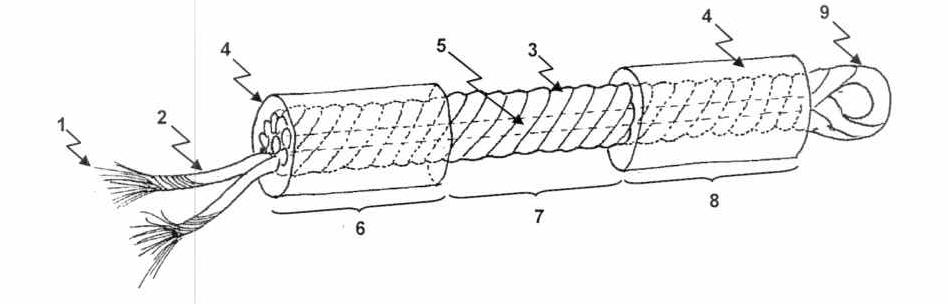
This diagram illustrates the three main components of the device: an elongated body (3) with two end parts: tibial (5) and femoral (6). The body of the device is formed from several twisted strands (2) of hydrogel threads (1), creating a rope-like structure. A hollow core (7) extends the length of the body, and an anchoring loop (9) sits at the femoral end. A tubular coating of hydrogel matrix (8) surrounds the tibial (5) and femoral parts (6), flanking an uncoated median section (4).
