This technology is an innovative microfluidic platform for potency testing of manufactured MSC to be used in developing therapeutics for immune and inflammatory conditions. Tests show it offers reproducible results via a highly functional, affordable design that is compatible with cell therapy manufacturing practices.
Georgia Tech’s high-throughput MSC potency assay is designed to allow for improved QC actions over the existing standards and permit controlled scalability at a reasonable cost. The QC metric that this technology addresses is the MSC immunomodulatory potential. The current standard tests MSC-mediated T-cell suppression in an MSC:PBMC (peripheral blood mononuclear cell) co-cultures planar assay. That assay does not provide a reproducible result, nor is it agile enough to handle increased sample volumes because of PBMC donor variability.
Georgia Tech’s new microfluidic platform successfully demonstrates a significantly higher correlation between the MSC secretory response within the microfluidic assay and the MSC response in vivo compared to traditional 2D cultures. Furthermore, the assay has an innovative microfluidic design, permitting as many as 40 devices to run simultaneously, and each chip’s estimated cost is 0.52 USD. No cleanroom or costly lithography techniques are used in the fabrication of the device. It is anticipated that the high-throughput MSC potency assay configuration could integrate effortlessly into existing cell therapy manufacturing practices, which would cut down the costs and improve outcomes of cell scale up.
- Reproducibility: The well-defined cellular environment of the device enables a highly reproducible MSC response not observed in 2D cultures.
- Scalable: The assay’s simplistic design is amendable to cost-effective scale up.
- Economical: The low cost of the microfluidic chip and the ease of implementing the assay into existing cell therapy manufacturing practices provide for low costs to enter the market.
- MSC manufacturing
MSC were discovered in the 1970s and have been of great interest to researchers for their potential as a therapeutic for immune and inflammatory indications. These cells historically have not had the same ethical discussions as embryonic stem cells. Many biotechnology companies have invested millions of dollars into the development of MSC therapeutics, but the U.S. Food and Drug Administration (FDA) has expressed considerable reservations to approving MSC therapies due to questions around quality control and scalability. As of 2020, there had only been 10 MSC therapies approved globally (none by the FDA), with a disproportionate 900+ clinical trials listed on the National Institutes of Health Clinical Trial online database. The Georgia Tech high-throughput MSC potency assay shows significant potential to address the FDA’s concerns with this emerging therapeutic market and bring more solutions to the patients in need.
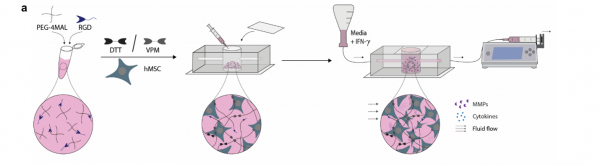
Development of microfluidic system informed from in vivo MSC secretion
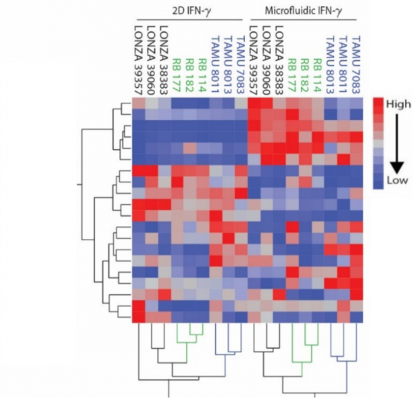
Two-way clustering heat map of analyte secretion across assay platforms
