Morbidity and mortality rates for single ventricle heart disease are high
Single ventricle heart disease is a rare type of congenital heart defect that affects roughly five in 10,000 newborns. Although single ventricle disease may be prevented in some fetuses by percutaneous transcatheter balloon valvuloplasty of the pulmonary or aortic valve, the valve eventually needs to be replaced when restenosis or regurgitation develops. On average, a valve in a child must be replaced in 7.5 years. When single ventricle disease cannot be prevented, the morbidity and mortality rates are high, and most patients will require a heart transplant.
Fully degradable heart valve scaffold implanted in fetus creates a living autologous valve
The highly regenerative potential of a fetus provides the perfect host to create a living neovalve from the fetus’s own tissue that can restore blood flow and heart valve function. The tissue-engineered scaffold of this bioresorbable heart valve serves as a template for tissue formation. As the scaffold degrades, the neovalve forms, creating a living autologous valve. This technology can potentially produce a valve that continuously grows with the patient and precludes the need for multiple heart valve replacements during the patient’s lifetime.
The scaffold is created using a biodegradable polymer material, such as polycaprolactone (PCL), and a biodegradable non-toxic metal alloy. Synthetic polymers like PCL, known for tunability and low cost, have long been used for cardiovascular applications, such as heart valve repairs and replacements. A prototype of the fetal valve was manufactured by laser cutting a resolvable ZnAl metal tube. The leaflets were constructed with a sutureless spray-on or dip-coat technique using the safe bioresorbable synthetic polymer PCL.
When combined with the latest in-utero surgical procedures, this cutting-edge polymeric valvular repair strategy may help restore healthy hemodynamics and mitigate many of the problems arising from congenital heart defects in children.
- Better patient outcomes: Replacing a stenotic fetal heart valve with a living autologous tissue engineered heart valve (TEHV) has the potential to correct the complex cardiac anomalies that cause single ventricle physiology.
- Fewer procedures: With this approach, the valve has the potential to accommodate patient growth and eliminate the need for multiple heart valve replacements during the patient’s lifetime.
- Safe materials: Polymeric materials can be mass produced for biomedical usages and have a proven record in cardiovascular applications, such as heart valve repairs and replacements.
- Minimal increase in procedural risk: The transcatheter nature of this valve deployment makes the risk similar to a traditional fetal valvuloplasty.
- Successful prototype: The feasibility of replacing the pulmonary valve with a fully biodegradable TEHV was demonstrated in a fetal lamb model using endovascular techniques.
This medical device can be used for the treatment of congenital heart disease (specifically single ventricle physiology) via in-utero repair and replacement of fetal heart valves.
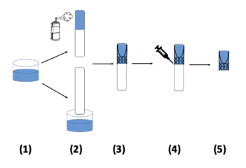
Fetal valve production. (1 and 2) The polymeric solution is solubilized before being either dip coated (bottom) or sprayed (top) onto the mold. (3) A frame is mounted onto the mold that is coated with the polymeric material and allowed to dry. (4) A second layer of the polymeric material that adheres to the inner layer is added outside of the valve frame. (5) The valve is allowed to dry again and finally removed from the mold.

Prototype laser cut fetal stent
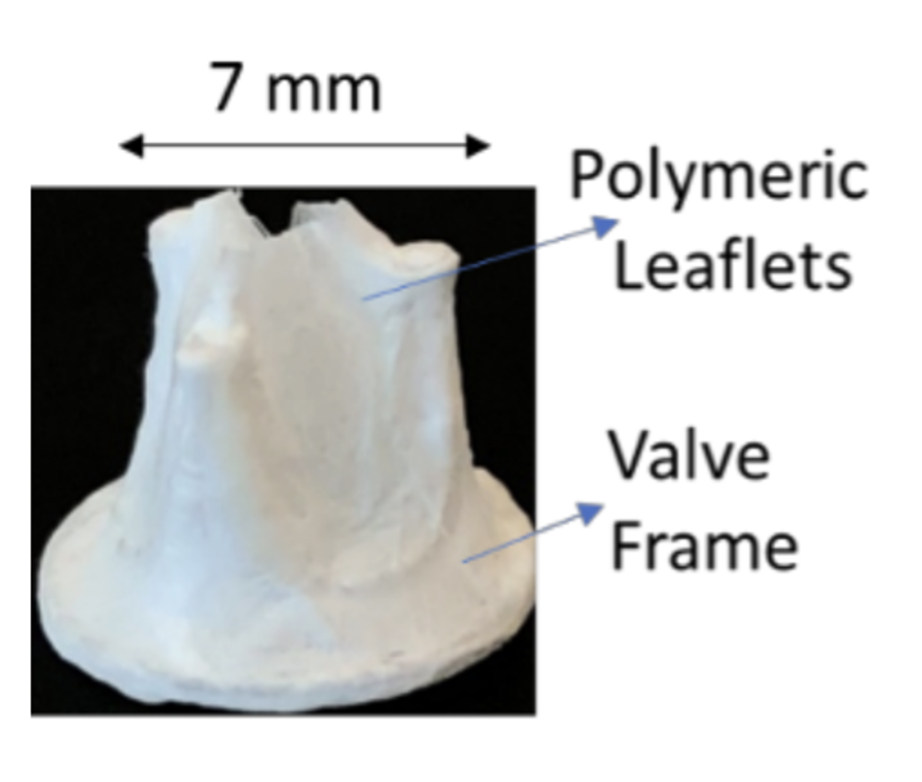
A dip-coated polymeric fetal valve
