This method deploys engineered synthetic biomaterials as a scaffold to form tumor immune microenvironments as tools to facilitate immunotherapeutic drug testing for cancer. Researchers at Georgia Tech developed this technology to address a testing platform gap in the immune oncology drug development pipeline. This technology has demonstrated tumor formation in 100% of injected animals, which is substantially greater than conventional biological matrix vehicles used for tumor implantation work. Additionally, the phenotype of tumor-infiltrating immune cells is highly consistent between formed tumors as well as testing batches. This infiltrating immune cell phenotype can also be modulated based on the composition of the engineered biomaterial matrix scaffold used to more closely mimic different immune responses observed in clinical samples.
Many current tumor model systems used for immune oncology drug screening are prohibitively expensive, highly variable, and slow, making them poorly scalable for large drug screening studies. The engineered tumor microenvironments developed by Georgia Tech aim to improve upon these systems by providing a chemically defined synthetic matrix that results in increased tumor formation rate, decreased disease latency, and diminished variability of immune infiltrates. By enabling immunotherapeutic testing relevant to the variability in tumor immune microenvironments seen in human cancer patients, this invention aims to deliver a robust, consistent, and scalable method for modeling immunologically defined tumors.
- Scalable: Enables increased tumor formation rate without sacrificing the dynamic complexity of an in vivo adaptive immune response
- Improved consistency: Demonstrates consistent induction of tumor growth, latency, and immune infiltration in early analyses of the technology
- Cost-effective: Eliminates the repetitive processes of conventional methods and leverages inexpensive hydrogel
- Widely applicable: Adaptable to multiple solid tumor types which currently lack immunotherapeutic strategies, which could help researchers better predict immune responses and develop novel, more effective anti-cancer treatments
- Research tools
- Pharmaceutical drug development
- Immunotherapies for cancer treatment
- Diagnostics
Immunotherapy has emerged as one of the most powerful anti-cancer therapy classes but is limited by existing preclinical models. The influence of differing immune microenvironments within tumors observed in clinical disease and associated with immunotherapeutic resistance cannot be manipulated to facilitate drug testing workflows without laborious, expensive approaches that yield inconsistent results. Due to their reliance on the coordinated effects of multiple arms of the immune system, immunotherapies are tested primarily using preclinical animal models.
While immunotherapies are one of the most promising cancer treatment tools, response rates in the clinic are disappointingly low. These poor outcomes reflect an unmet need in understanding the differences in patient responses and a demand for tools to develop new and better immunotherapeutic strategies for patients who are less likely to respond to immunotherapies.
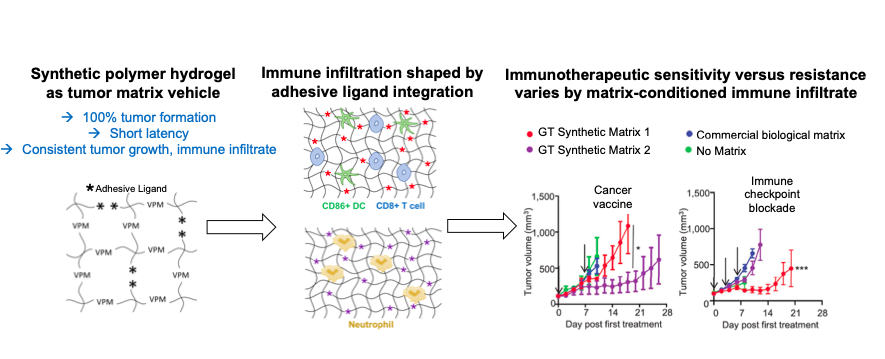
Engineered hydrogels produce consistent tumor immune microenvironments that control tumor growth and response to immunotherapy.
