Lateral flow assays (LFAs), or rapid tests, such as common pregnancy or drug tests, typically attempt to identify the presence of a substance via a one-time chemical reaction using a paper-based assay, a liquid (e.g., blood, urine, saliva, etc.), and a reagent. However, more complex diagnostic testing, such as COVID-19 detection, can require multi-step, multi-reagent chemical reactions with specific timings. Georgia Tech researchers have developed a technique to control capillary flow on paper-based LFA substrates that leverages a gradual process, thereby allowing for multiplex molecular detection. This technique has led to the development of low-cost, point-of-care (POC) toolkits for the diagnosis of SARS-CoV-2 and influenza A and B viruses, as well as improved pregnancy testing.
Researchers were able to create “timers” by imprinting roadblocks on the flow path with water-insoluble material and utilizing the gradual formation of a void between a wetted paper and a sheath polymer tape (i.e., delamination). Timers are drawn on paper at strategic nodes to hold the capillary flow for a desired period of time, hence multiple liquids can be introduced into the reaction following a programmed sequence. Furthermore, by marking the flow boundaries with a material that remains permanently adhered to the sheath tape, researchers were able to imprint on paper complete LFAs programmed to execute multi-step chemical reactions in an optimized manner. Using this technique, Georgia Tech developed 1) an LFA with built-in signal amplification to detect human chorionic gonadotropin (i.e., a pregnancy test) with an order of magnitude higher sensitivity than the conventional assay and 2) a device to extract and purify DNA from blood, saliva, and urine samples without relying on laboratory instruments for post-processing.
To see more technologies like this by Dr. Sarioglu and his team, please click here.
LFAs offer unique advantages, such as rapid analysis, low-cost, portability, ease-of-use, instrument-free operation, and compatibility with various biological samples (e.g., blood, plasma, serum, urine, sweat, and saliva). These characteristics make LFAs the predominant diagnostic format for POC applications. The addition of delaminating timers has the potential to greatly increase the diagnostic capabilities of LFAs. This technology also includes a mathematical model to guide the design of various flow sequences and timings.
- ASSURED: LFAs readily satisfy the majority of the ASSURED (affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free, and deliverable to end-users) criteria reported by the World Health Organization to establish capabilities of POC devices.
- Agile: The addition of timers to LFAs provides an opportunity to develop more complex multi-step testing, as well as additional benefits such as built-in signal amplification and POC DNA extraction and purification.
- Automated: The ability to sequentially deliver different reagents into a reaction via programmable timers imprinted on paper makes it possible to automate multi-step assays that otherwise could only be performed in laboratories or with manual intervention.
- Advanced: The capabilities provided by this technology can make it possible to develop LFAs that can rival ELISA- or PCR-based assays for sensitive and specific detection of pathogenic targets such as Zika virus, human immunodeficiency virus (HIV), hepatitis B virus, or diseases like malaria.
- Clinical analytical assays
- Pathogen detection
- Point-of-care diagnostics
- At-home diagnostic test
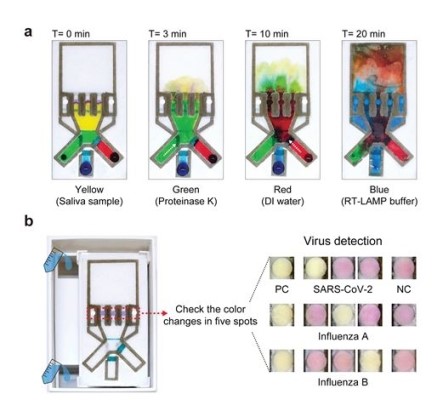
Figure 1. COVID-19 and flu saliva test on paper: a) The automatic sequential delivery of multiple reagents required for virus test; b) Water pouring into the device triggers the virus assay, allowing the presence of SARS-CoV-2 and influenza A & B viruses to be visually identified by the color changes in the corresponding detection spot
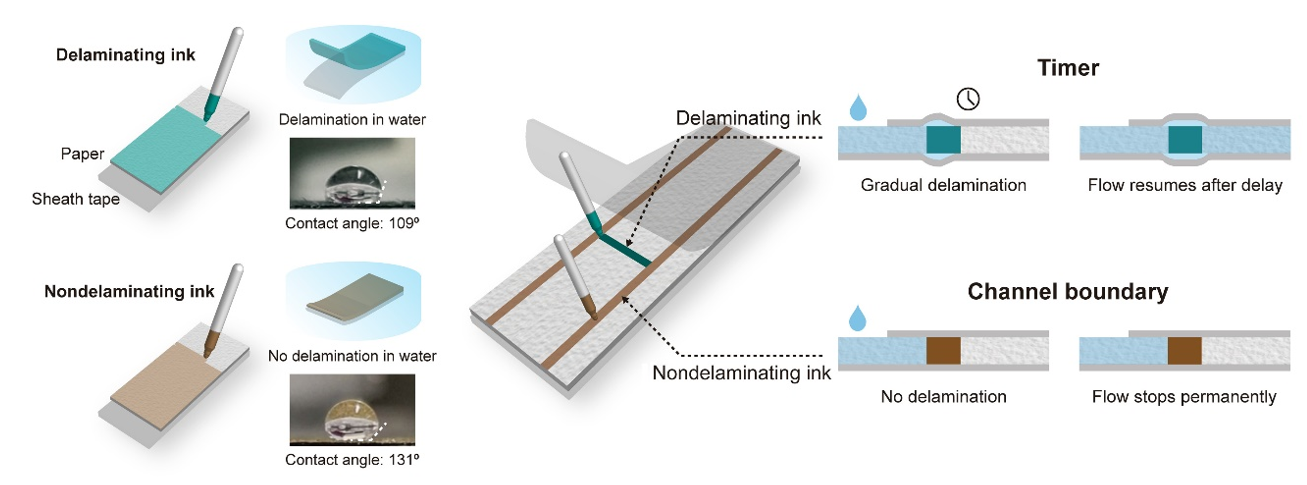
Figure 2. Capillary flow control via sheath tape delamination: Manipulation of capillary flow on paper by imprinting patterns to modulate the paper-tape adhesion. The timer is drawn with a water-insoluble ink that is free of hydrophobic resin (delaminating ink). When the paper is wetted, a void gradually forms at the paper-tape interface and eventually resumes the flow. The flow boundaries are defined with a water-insoluble hydrophobic ink (non-delaminating). The resin remains attached to the tape and permanently blocks the flow.
Photo Credit: Dohwan Lee, Georgia Institute of Technology.
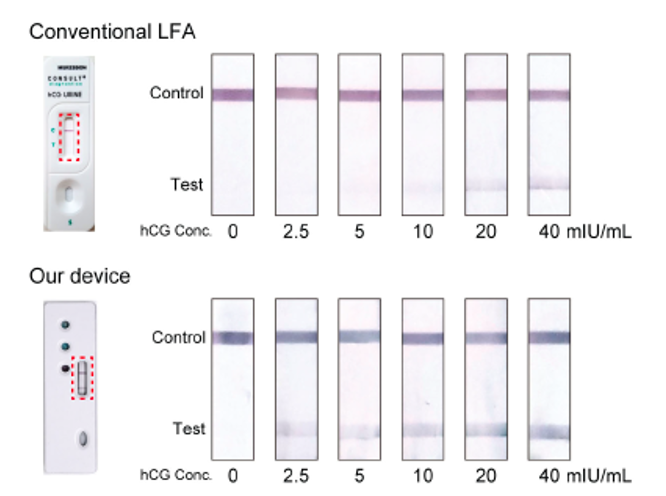
Figure 3. Assay results corresponding to samples with different human chorionic gonadotropin (hCG) concentrations obtained using the conventional LFA and the Georgia Tech device.
