This method creates active isomers of high-demand atomic metals such as gold (Au) and palladium (Pd) using significantly less precious metal (by a factor of one to ten thousand) and exhibiting superior catalytic performance. Georgia Tech researchers have used an atom-by-atom deposition method (“bottom up”) to prepare atomic clusters of Au1Pd2 and Au2Pd1 in a stable isolation matrix (polyaniline [PANI]), rather than starting from a large mass of material and then decreasing its size until reaching the atomic level (“top down”). This approach allows for the precise ordering of the metal atoms, which can result in more efficient catalysts with distinct, quantized catalytic properties.
The sequence in which individual atoms are added affects the final structure of the cluster, which, in turn, affects its catalytic activity. One of the isomers created (PANI-Pd-Pd-Au) exhibited approximately five times the activity of the most active homo-atomic clusters of Au or Pd (i.e., PANI-Au6 and PANI-Pd2). The activity of the resulting hetero-tri-atomic clusters was demonstrated in the electrochemical oxidation of alcohols—as a potential application to devices such as fuel cells—and has the potential to allow for the creation of highly tailored atomic clusters.
- Innovative: Generates new isomers by changing the order in which atoms are added in a hetero-atomic cluster (can maximize available catalytic sites)
- Efficient: Creates and optimizes active isomers of atomic metals such as Au and Pd, increasing the catalytic efficiency of these high-demand metals (requires minimal precious catalytic metal)
- Scalable: Provides a scalable process resulting in theoretically predictable catalytic properties
- Accessible: Employs an existing, cost-efficient electrochemical screening processes to assess the catalytic efficiency of new atomic constructs
The ability to create highly tailored atomic clusters would suit various chemical and pharmaceutical industry applications, as well as provide a method for the electro-oxidation of alcohols, with applications such as:
- Fuel cells (vehicles, stationary and portable power, off-road applications, marine vessels, consumer electronics, direct fuel cells, etc.)
- Gas sensors (carbon monoxide, oxygen, lower explosive limit, alcohol, etc.)
Optimizing the efficiency of high-demand, precious-metal catalysts could have a wide impact across numerous industries:
- Automobile
- Pharmaceutical
- Refinery
- Alternative energy
- Medical
- Electronics
Properties, preparation, and practical applications of metal catalysts depend on their size. A nanometer-size cluster, for example, contains millions and millions of atoms. Such clusters consist of active surface atoms while the bulk of the atoms are inactive.
The first atom deposited in this process effectively anchors the cluster to the matrix, while the activity is dictated by the next two atoms deposited. It was found that the highest activity (towards electrooxidation of n-propanol in alkaline medium) was for clusters that had Pd and Au deposited second and third, respectively. By having a point of attachment as a geometric reference point, these hetero-clusters have more isomers than gas phase clusters, which gives rise to different activities.
Atomic clusters consisting of more than three atoms in a hetero-cluster will have an even larger number of possible isomers. It is already evident that electrochemical screening is a good, cost-effective way to assess the catalytic efficiency of these new atomic constructs, which could help expand how this “ordering effect” can be utilized in the future. Additional theoretical work could provide useful guidance for greatly simplifying the optimization process. The observed results are in good agreement with the few available theoretical calculations of the HOMO-LUMO gap energy of these structures.
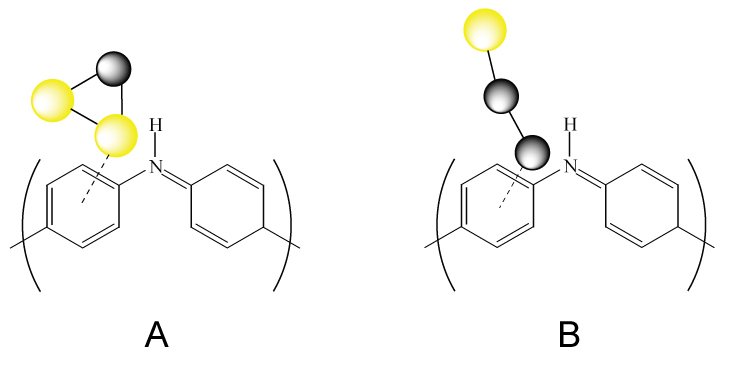
Illustration of the structure of A) triangular and B) linear clusters added to the imine site of a PANI chain. Yellow represents gold and gray represents palladium.
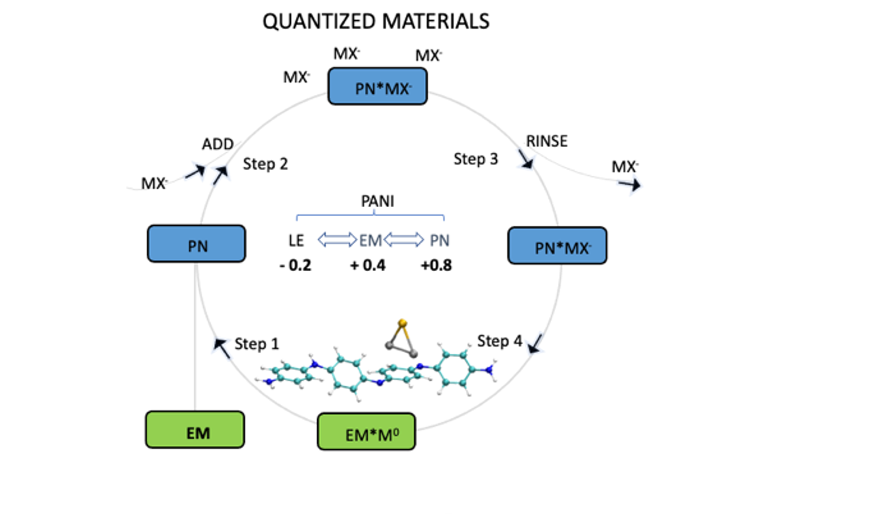
Sequential preparation of quantized materials: PN, EM, and LE are oxidized, semi-oxidized, and reduced forms of polyaniline (PANI), respectively, and MX stands for metal precursor complex. The molecular structure inside the circle is a repeat unit of polyaniline and the triangle represents cluster consisting of two atoms of Pd and one atom of Au, as an example of an atomic cluster.
