New antibodies allow for precise and reproducible detection of folded myocilin using recombinant DNA technology to aid in glaucoma research and development of clinical diagnostics. By leveraging their understanding of the unique branched architecture and aggregation propensity of glaucoma-associated myocilin, researchers at Georgia Tech have created antibodies that selectively bind to identified epitopes within myocilin only when it is in its native conformation. This provides significantly more insight than currently available commercial antibodies that detect a mixture of folded, cleaved, and misfolded myocilin. The recombinant aspect of these antibodies also explicitly addresses concerns from the National Institutes of Health over authenticity of research reagents and reproducibility.
- Exclusive detection: Unlike current myocilin-directed antibodies that do not differentiate among numerous disease-associated states of the protein (e.g., folded, cleaved, and misfolded myocilin), these reagents only detect folded myocilin
- Authentic and reproducible: Antibodies are produced using recombinant DNA technology, which enables robust precision sequencing of the reagent and maintains fidelity over time
- High affinity: Antibodies detect both mouse and human myocilin with well-characterized epitopes and high affinity
- Research tool
- Glaucoma research and diagnosis
- Research on the anterior and posterior eye
- Research validating trabecular meshwork cell lines
Primary open-angle glaucoma is the second leading cause of irreversible blindness, affecting four percent of the population over age 40—about 70 million people worldwide. Glaucoma-associated myocilin is present at relatively high concentrations in the trabecular meshwork, a tissue in the anterior eye segment that is closely linked to maintaining eye pressure. Five percent of glaucoma cases are linked to a gain of toxic function mutations in myocilin, which cause misfolding and aggregation and lead to increased intraocular pressure and damage to the eye.
Therefore, differentiating among different states of myocilin (full-length, cleaved, or misfolded) is important broadly in the context of glaucoma. Current research uses epitopes that are recognized by myocilin-directed antibodies but do not differentiate among the numerous disease-associated states of the protein. This limits insights that can be gleaned from the many types of experiments that employ such reagents. George Tech’s innovation leverages a structural understanding of myocilin’s uniquely branched architecture and aggregation to create new antibodies that selectively bind to a folded epitope of myocilin.
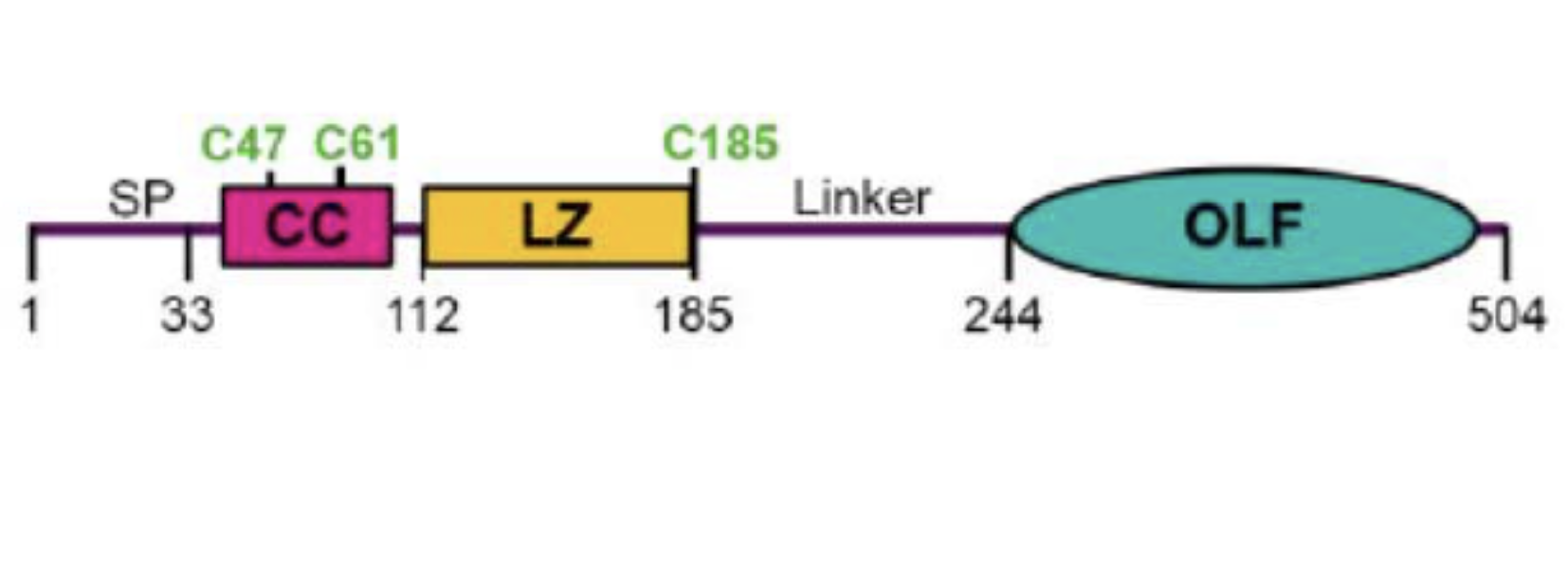
The gene structure depicting the domains of myocilin, including signal peptide, location of key cysteine residues, and its coiled-coil, leucine zipper, and olfactomedin domains.
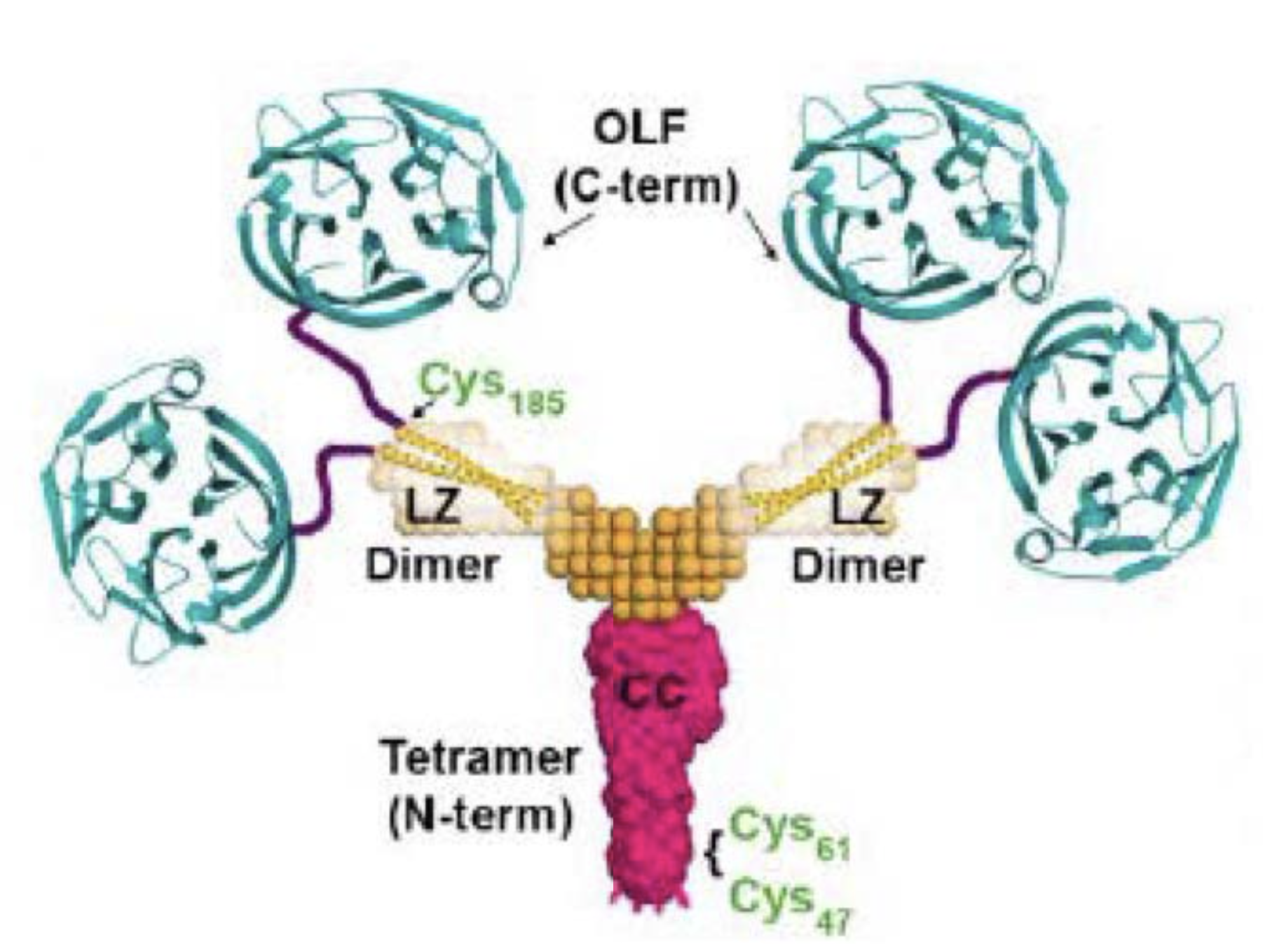
The myocilin quaternary structure based on solution X-ray scattering, X-ray crystallography, and chemical cross-linking experiments.
