This transcatheter heart valve (THV) incorporates innovative design features and uses a new multi-arm mechanism to displace native heart valve leaflets. It also aids in proper THV deployment and positioning during or in preparation for transcatheter aortic valve replacement (TAVR). Georgia Tech’s technology addresses a critical need identified by the U.S. Food and Drug Administration (FDA) to decrease risk factors associated with thrombosis development in TAVR by helping decrease flow stasis, preventing coronary obstruction, and optimizing THV deployment.
TAVR candidates number approximately 270, 000 patients across North America and the European Union, underscoring the potential impact of further advances in this relatively new treatment for patients with severe, symptomatic aortic stenosis. Reduction of complications such as thrombotic events following TAVR would reduce patient hospitalizations and healthcare resource utilization and improve patient outcomes.
Georgia Tech’s innovation provides three main design advantages, all of which help to minimize the risks associated with TAVR and optimize patient outcomes:
- Reduced flow stasis: This mechanism displaces the native valve leaflets away from the THV stent frame, essentially removing its “cover” and allowing blood to move freely through the open stent cells. This can help to significantly reduce flow stasis in the neo-sinus region, thereby reducing thrombus initiation/progression.
- Prevention of coronary artery obstruction: Coronary artery obstruction following TAVR is a rare but often fatal complication. In rare cases in patients with unusually large native leaflets or small aortic roots, the large leaflets block the flow of blood to the coronary arteries as the new valve’s scaffolding opens. Georgia Tech’s design displaces the native valve leaflets away from the THV stent frame, which can help to reduce this risk.
- Optimized THV deployment: The innovation offers dual feedback to aid clinicians in determining optimal THV deployment height—a key factor in optimizing patient outcomes. As the device’s “arms” extend against the native vessel wall, they create both tactile feedback during THV positioning as well as visual feedback via fluoroscopy. In addition to deployment height, the multi-arm design supports axial alignment of the THV with respect to the native vessel wall—a factor shown to impact valve performance.
Georgia Tech’s innovation can be:
- Developed as a new THV device
- Incorporated into currently available THV devices
- Incorporated into new THV device designs
- Developed as a fitting and implanted prior to THV implantation
Aortic stenosis (AS) is the most prevalent valvular heart disease in developed countries and is associated with high mortality when left untreated. Patients diagnosed with moderate or severe AS have historically undergone surgical aortic valve replacement (SAVR); approximately 200,000 surgeries are performed annually worldwide. In recent years, TAVR has emerged as a safe and effective alternative treatment for patients with severe, symptomatic AS and who are deemed intermediate or high surgical risk. TAVR is a non-surgical (percutaneous) approach to aortic valve replacement which was first successfully performed in a human in 2002. Since 2012, approximately 500,000 TAVR implants have taken place worldwide.
How it Works
Georgia Tech’s mechanism can be incorporated into the design of the THV stent itself, with multiple “arms” that automatically extend outward near the level of leaflet insertion. As the THV is moved into position, the arms engage the native valve leaflets, pushing them back and folding them towards the bottom of their respective native sinuses. The arms also flatten out, providing both tactile and visual feedback to indicate when the THV is properly positioned.
This multi-arm mechanism is designed to conform with current THV deployment procedures, adding only slight modifications to allow for the arms’ expansion before the valve is positioned and fully deployed. In most cases, this device should not increase the amount of time required for THV replacement procedures.
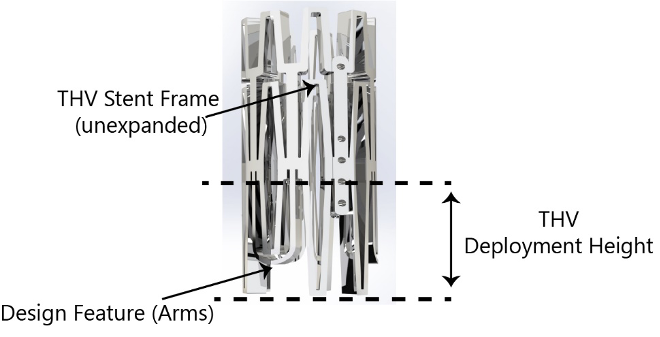
Georgia Tech’s multi-arm mechanism in its unextended position.
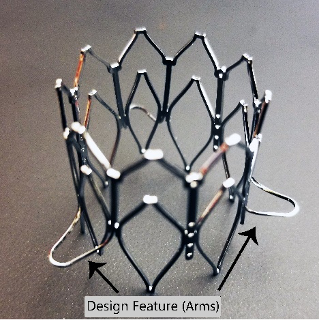
Georgia Tech’s mechanism with arms extended. The arms must extend far enough to create a landing zone larger than the native annulus to allow for efficient THV deployment.
