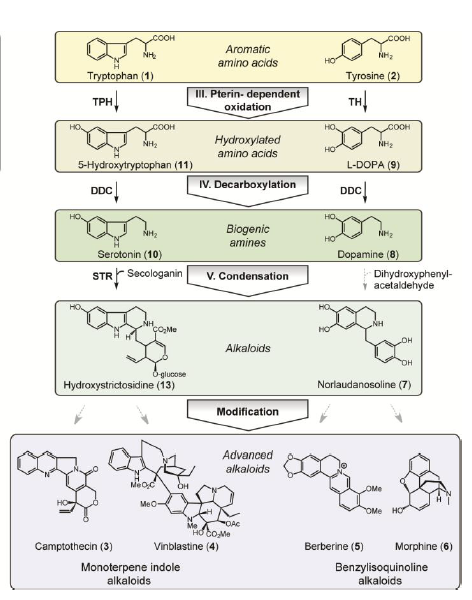
Inventors at Georgia Tech have developed a yeast strain that uses the pterin-dependent amino acid mono-oxygenase pathway to perform the amino acid hydroxylation step. Key to this strategy is development of a yeast strain that produces the co-factor for amino acid mono-oxygenase, tetrahydrobiopterin (BH4). The inventors have used this strain to synthesize serotonin and dopamine with yields of about 0.3 mg/mL. Then, by including a codon-optimized gene for strictosidine synthase, they were able to use the strain to produce hydroxystrictosidine.
This technology can be used in the development of yeast-based syntheses of plant alkaloids, or any other biosynthetic pathway that uses 5-hydroxytryptohan or L-DOPA as precursors. It can be used for the synthesis of compound monoterpene indole alkaloids derived from strictosidine (e.g. quinine, camptothecin, ajmalicine, serpentine, vinblastine and vincristine) or benzylisoquinoline alkaloids derived from dopamine (e.g. berberine and morphine).
- Yeast strains available for immediate deployment
- Also applicable to production of pyrrolizidine alkaloids (based on citrulline)
- Yields of ~ 0.3 mg/mL in liquid culutre
Plant alkaloids, in particular those derived from tryptophan and tyrosine, are important for their medicinal uses as anticancer, antimicrobial, and analgesic agents. Due to their complex structures and chiral centers, it is often easier to isolate alkaloids from plants than to synthesize them and separate them from enantiomeric impurities. However, they make up only a small fraction of a plant’s biomass, and despite efforts to breed or engineer plants with higher yields of these valuable compounds, their isolation remains costly. A microbial synthetic route would provide numerous advantages over isolation from plants: fast and scalable growth, rapid extraction from the growth medium, an absence of similar products, and lack of feedback inhibition. A critical first step in the synthetic pathway is hydroxylation of amino acids tryptophan and tyrosine to 5-hydroxytryptophan and L-DOPA. Other research groups have used cytochrome P450s to accomplish this, with only moderate success, due to the propensity of P450s to be promiscuous with their oxidation substrates.