This novel methodology is designed to produce antimicrobial peptide (AMP) prodrugs with the ability to self-titrate in the presence of disease-related enzymatic activity. The prodrug includes a cationic AMP locked by anionic peptides and a protease-cleavable linker. Upon proteolytic cleavage of the substrate, the prodrug unlocks to release AMP, creating the mechanism for auto-titration. Release of the prodrug is only activated by the presence of bacteria proteases—for example, E. coli outer membrane protease T (OmpT)—and increased concentrations of bacteria activate higher concentrations of prodrug. In the absence of bacteria, the prodrug remains inactive and inert, eliminating the risk of off-target toxicity.
Georgia Tech researchers modeled bacteria-prodrug dynamics and identified a dimensionless quantity—the Bacterial Advantage Heuristic—that governs the success or failure of a prodrug, depending on enzymatic activity and bacteria growth. This approach has the potential to spur the development of new prodrugs and improve the clinical administration of existing prodrugs, contributing to the reduction of antibiotic resistance.
- Effective: Activates an internal dosing mechanism such that the magnitude of an infection locally determines the prodrug dose
- Robust: Increases the stability of the prodrug form by neutralizing the effective charge of the administered antimicrobial peptide
- Improves safety: Decreases the potential for off-target effects
- Preventative: Mitigates the risks for development of drug-resistant bacteria
- Antimicrobial peptide-based therapeutics
- Research strategies for prodrug administration
- Research tool to support antibiotic-resistance research
Antibiotic success is markedly improved by proper titration of drugs, as overdosing leads to off-target toxicity and underdosing increases the likelihood of pathogens’ developing resistance. However, optimal drug doses are difficult to achieve over the course of treatment because the infection burden changes dramatically over time. Prodrugs, which represent approximately 10 percent of all FDA-approved drugs in the last decade, are a promising solution because they are automatically titrated by a disease-related activation mechanism, increase bioavailability, and reduce the risk of off-target effects.
Bacteria-activated prodrugs are an emerging strategy to treat antibiotic-resistant infections. This Georgia Tech research has the potential to provide strategies for preventing resistance mechanisms in new and existing prodrugs.
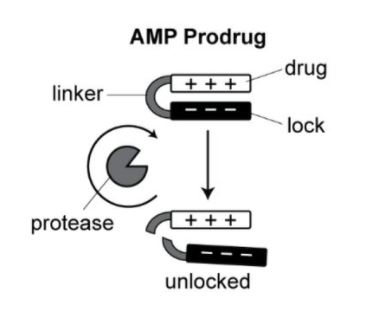
A cationic AMP drug locked by an anionic peptide with a protease-cleavable linker is activated by OmpT protease activity.
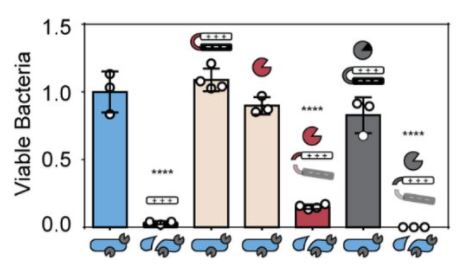
Bacteria viability assay quantifying drug toxicity relative to untreated bacteria control (blue bar). Positive control for AMP toxicity (black bar). Negative control for locked AMP or Tobacco Etch Virus (TEV) protease alone (tan bars). Positive control for TEV protease with locked AMP (red bar). Negative control for locked AMP with OmpT inhibitor, aprotinin (grey bar). Experimental condition of bacteria treated with locked AMP activated by natively expressed OmpT (white bar). gtr
