This “salinity exchange” technique has the potential to greatly increase public drinking water supplies using otherwise unusable wastewater—all while greatly reducing energy consumption, costs, and environmental impacts. Treating domestic wastewater for use as fresh drinking water is more economical than desalination processes, but public acceptance and safety concerns are major obstacles. Georgia Tech’s new salinity exchange process overcomes those obstacles, producing potable water from seawater or brackish water by transferring its salt content to domestic wastewater. This creates high-salinity wastewater that is treated and discharged into the ocean and lower salinity seawater or brackish water that is easily treated for public use.
- Increases water supply: This process takes advantage of abundantly available wastewater to produce clean drinking water.
- Requires significantly less energy: Salinity exchange uses much less energy than conventional desalination technologies for which energy consumption is still a major obstacle. Georgia Tech’s bench scale prototype has demonstrated energy consumption of less than one-third of conventional reverse osmosis.
- Publicly acceptable: Drinking desalinated seawater and brackish water is much more cognitively appealing than direct potable reuse of treated wastewater, overcoming public perception obstacles to water treatment.
- Lower environmental impact: Because salt removed from the seawater and brackish water is diluted by the low-salinity wastewater, the process does not generate brine that can cause environmental and ecological damage.
This new process for production of potable water is most applicable in coastal areas where seawater or brackish water is readily available and wastewater is routinely discharged into the ocean after treatment.
Population growth and industrialization have rendered water scarcity an increasing concern in recent decades. Because seawater and brackish water account for about 98% of all the water on the earth, desalination of seawater and brackish water has been an important strategy to address the ever-increasing global demand for fresh water. While there are several types of desalination processes in use, they have high energy requirements and many produce greenhouse gases.
Potable water reuse, particularly direct potable reuse (DPR), is increasingly being considered a large untapped resource to help mitigate the exponentially growing water demands. Public perception of drinking treated wastewater, however, is a big barrier. Georgia Tech’s salinity exchange process makes DPR an acceptable option for public drinking water.
This technology could impact more than 1,400 coastal wastewater treatment plants that serve over one third of the U.S. population and discharge approximately ten billion gallons of treated effluent per day.
How It Works
This salinity exchange process is a synergetic integration of desalination, extraction of salinity-gradient energy, and water reuse. Salinity exchange electrodialysis (SE-E) is an integrated system of reversed electrodialysis and electrodialysis. Seawater and wastewater are separated by selective ion exchange membranes, while the transport of salts is driven by the combination of Donnan potential (the inability of certain ions to diffuse out from one phase or region to the other in water-based systems) and electric potential. This process can run both in batches and continuously.
Salinity exchange has been successfully achieved between influents of different salinities under various operating conditions as part of a comprehensive energy efficiency assessment. Laboratory-scale SE-E systems can produce high-quality desalinated water at 1 mL/min with an energy consumption less than 1 kWh/m3— or one-third of conventional energy consumption.
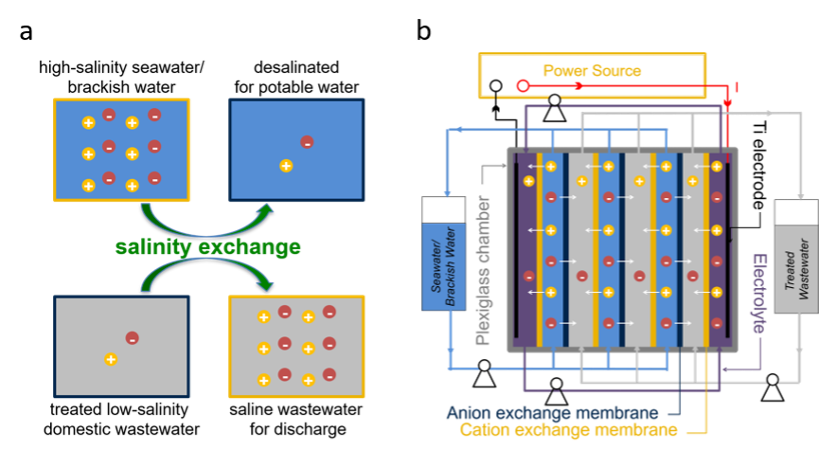
Salinity exchange a) Schematic of the salinity exchange concept and b) operation of a salinity exchange electrodialysis (SEE) system.
