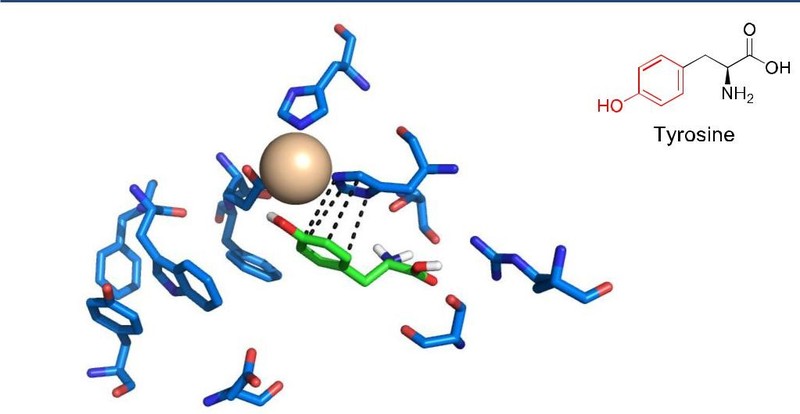
Inventors at Georgia Tech are using a natural enzyme, specifically a pterin-dependent aromatic amino acid mono-oxygenase in vivo in hopes of modifying lignin monomers. The purpose is to develop hydroxylation processes for use on lignin based aromatic monomers that could eventually be applied at the industrial level. In nature, this enzyme hydrolyzes amino acids with structural similarity to relevant lignin monomers. Pterin, a complex compound, acts as a cofactor in the reaction. Inventors believe that this enzyme could successfully modify at least two lignin-derived aromatic monomers, including the hydrolyzation of vanillin to produce a higher value intermediate, vanillic acid. This invention could provide a valid route to biologically modifying lignin related aromatic monomers into feedstock for a wide variety of industrial applications.
- Could provide substantial high value intermediates
- Enzymatic catalysis is more energy efficient, yields less (industrial type) waste than chemically based methods
- Higher level of precision than most chemical reactions
- Process may be renewable- minimal waste and environmentally sound
- Fuels
- Pharmaceuticals
- Components used in foods
- Aromatic-based feedstocks-
- Plastics, fuel alternatives, agricultural chemicals, resins
Aromatic monomers are important precursors to valuable industrial chemicals, pharmaceuticals, biofuels, and other applications. They are typically generated through various types of catalysis in industrial, chemical-based facilities. Selective monomer hydroxylation could convert these precursors to valuable feedstock for these and many other applications.