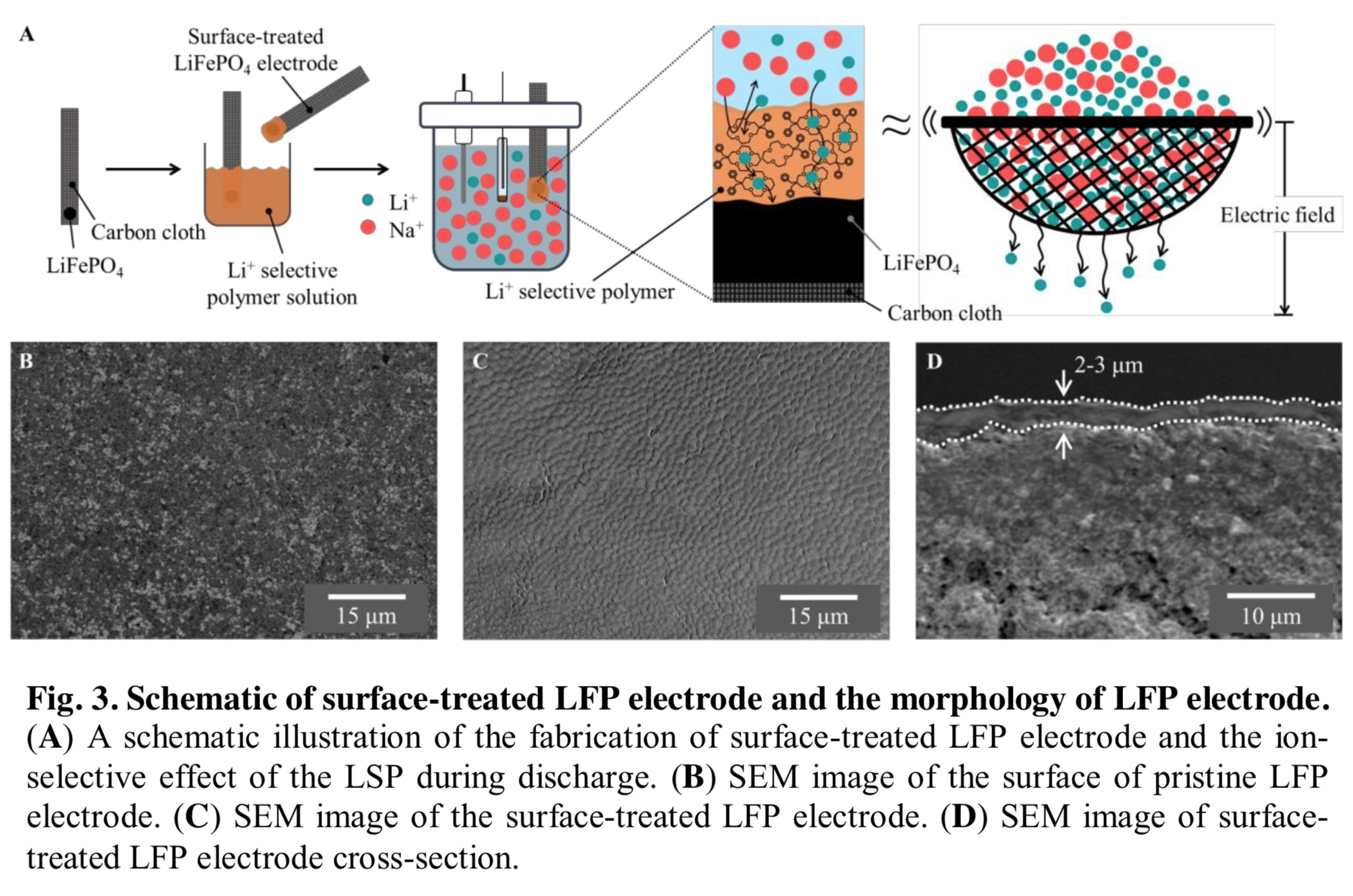
Taking advantage of the differing electrochemical characteristics of lithium (Li+) and sodium (NA+) ions, researchers at Georgia Tech are extracting lithium from seawater electrochemically using a high lithium-selective polymer sieve on a lithium iron phosphate (LiFePO4) electrode.
More than 99.9% of the world’s lithium is found in seawater but in dilute concentrations. Extracting lithium is, therefore, difficult and requires a highly selective process because of the high concentration of sodium ions in seawater. Georgia Tech’s electrochemical extraction method increases lithium selectivity by using crown ethers to synthesize a 0.09-mm thick lithium-selective polymer (LSP) membrane, which acts as an ion sieve to allow lithium to pass while blocking other ions. Their surface-treated lithium ferrophosphate (STLFP) electrode has innate properties of lithium-ion selectivity, a fast cycle life, and low environmental impact.
Georgia Tech’s STLFP and polymer sieve demonstrate great selectivity. Recovered solution has a molar ratio of Li+:Na+ enhanced from 0.2 to 2.15 after just one 2-hour cycle. This method provides a fast and practical electrochemical extraction method for lithium mining from seawater.
- Fast: This method shortens the time for lithium extraction from seawater from years to days.
- Energy-efficient: The STLFP has outstanding electrochemical storage properties and working potential, which can achieve high energy efficiency and obtain great lithium recovery.
- Eco-friendly: The recovery process requires no additional heating or chemicals.
- Highly selective: The innovative sieve allows Li+ to pass through while blocking Na+.
- Harvesting lithium from seawater
- Industrial-scale electrochemical mining systems
Lithium-ion batteries (LIBs) have been used extensively in consumer electronics, automotive, medical, and industrial markets since the 1990s. Recently, the rapid development of portable electronic devices and electric vehicles has led to an unprecedented demand for LIBs. Therefore, lithium has become a strategically important commodity and is one of the most valuable resources in the foreseeable future. The current lithium production levels, however, cannot satisfy the spiking demand and have been reported to cause negative impacts on the environment.
Georgia Tech’s innovation addresses this challenge and provides a faster, stable, and environmentally friendly strategy for mining lithium from the ocean.

Po-Wei Huang synthesizing lithium-selective polymer at the Georgia Institute of Technology. Photo by Haochen Yang

Electrochemical lithium extraction cell. Photo by Po-Wei Huang