Existing HIV pre-exposure protections lack effective tissue concentration and longevity at the most common virus introduction site
The female reproductive tract (FRT) is the most common site of HIV introduction, where the virus rapidly permeates the vaginocervical lining, reaching systemic lymph nodes within 24 hours. As pre-exposure protection (prEP), broadly neutralizing antibodies (bNAbs) are effective against a wide range of high-potency HIV strains, but they are currently administered parenterally (e.g., intramuscularly or intravenously or) – a method that does not deliver adequate antibody concentration or longevity in the FRT. Neutralizing antibodies also have several limitations: cold storage requirements for stability, high costs that may affect compliance, potential side effects and no protection against sexually transmitted diseases.
mRNA technology delivers a patient-friendly, safe, and effective HIV prophylaxis solution
The vaginal mucosa has a large surface area, considerable vascularization, and good permeability. In addition, delivering therapeutics to the vaginal mucosa bypasses liver metabolism issues and allows for self-application. These factors indicate that prevention therapy, or prEP, should focus on virus uptake inhibition in the lower FRT to prevent systemic virus dissemination.
This unique technology addresses the limitations of systemic administration of bNAbs by offering an intervention that is locally applied to the FRT. It comprises synthetic messenger RNA (mRNA) with a coding sequence containing the HIV-neutralizing monoclonal antibody PGT121 that is modified with a glycosylphosphatidylinositol (GPI) membrane-anchor coding sequence at the heavy chain nucleotide sequence of PGT121. Using the GPI anchoring mechanism ensures the FRT will retain the bNAbs for up to 30 days, providing a patient-friendly therapy option to enhance compliance.
This antibody therapy is aerosolized in water and locally administered to the FRT epithelia. The use of water as a carrier circumvents the possibility of local FRT irritation and inflammation often encountered with typical mRNA carriers.
This technology offers a safe and effective delivery system for providing patient-compliant HIV prophylaxis therapy to the FRT, addressing the myriad issues that affect prEP protocol adherence.
- Effective: Easy local application ensures mRNA is absorbed directly into the most common site of HIV introduction, potentially enhancing patient compliance and avoiding systemic complications.
- Long-lasting: The mRNA generates antibodies along with an anchoring sequence that retains the neutralizing antibodies in the FRT, providing month-long HIV protection.
- Safe: This delivery system uses water as a carrier, which prevents inflammation that may occur with traditional mRNA carriers. Additionally, local application bypasses systemic complications, such a first-pass metabolism by the liver, and adverse effects.
- Less expensive: This water-based, easy-to-administer therapy may reduce the need for repeated clinic visits, resulting in lower medical costs for patients.
- Stable: Using water as a carrier provides a more stable delivery system compared with parenterally administered products that require cold storage for stability.
- Translatable: This technology has been tested in non-human primates with positive results.
- Prevention of HIV transmission via the female reproductive tract
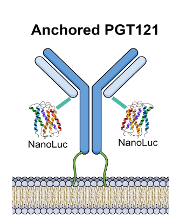
Schematic of membrane-anchored PGT121 using a GPI anchor fused to the three prime end of the heavy chain nucleotide sequence.
