Younan Xia and Shuifen Xie from the School of Biomedical Engineering at Georgia Tech have developed a method of synthesizing spatially controlled palladium (Pd)-rhodium (Rh) hetero-nanostructures with a core-frame structure and concave faces by confined overgrowth of Rh atoms at the corners and edges of Pd cubic seeds. This is achieved using a combination of kinetic control and blocking effect of bromide capping agent through a seed-mediated approach.
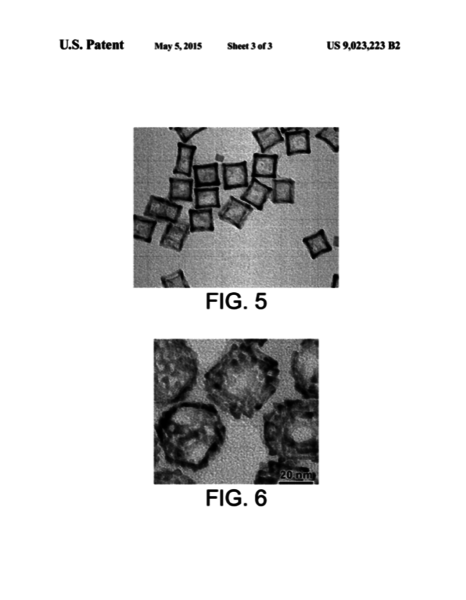
Three types of Pd polyhedrons (i.e., cuboctahedrons, octahedrons, and cubes) were applied as the seeds. Injecting Rh precursor solution slowly into a reaction solution containing the Pd polyhedral seeds and bromide capping agent with a syringe pump confines the generated Rh atoms to selectively deposit at the corner and edge sites of Pd cubic seeds or on the {111} surface of the Pd cuboctahedral and octahedral seeds. Using Pd seeds with different shapes, different Pd-Rh hetero-nanostructures with spatially controlled Rh distribution can be obtained. Transmission electron microscopy studies of the Pd-Rh cubic nanocrystals show that the products were constructed from Pd cubic cores and Rh nanoframes with both Pd {100} and Rh {110} planes being exposed on the surface, suggesting great potential in catalytic applications.
- Improved performance: Core-frame structure enhances shape stability and catalytic activity at high temperatures.
- Cost effective: Less rhodium is used than with other crystal growth methods.
- Simple: Crystals are formed in a scalable solution-based process.
- Versatile: The process can be used with other platinum group metals, including platinum, iridium and palladium.
- Catalysis, ideally designed for conversion of exhaust emissions into less harmful gases
- Plasmonics
- Biomedicine
- Photonics
- Sensors
- Electronics
This technology offers a method for synthesis of bimetallic core-frame nanocrystals.Bimetal nanocrystals are central to the development of catalysts for use in fuel cells, catalytic converters, and numerous industrial processes. The catalytic activity, efficiency, and selectivity of noble-metal nanocrystals are strongly dependent on their size, shape, composition, structure, and arrangement of atoms on the surface. A concave surface is of particular interest and importance for catalytic applications because of the involvement of high-energy facets and thus the high density of atomic steps and kinks.
Regulating the composition and structure of bimetallic nanocrystals in solution phase is challenging. It is difficult to obtain controlled, synchronous nucleation and growth of both metals due to their intrinsic difference in chemical reactivity. Georgia Tech’s innovative process offers a new method for growing these structures easily and cost effectively.