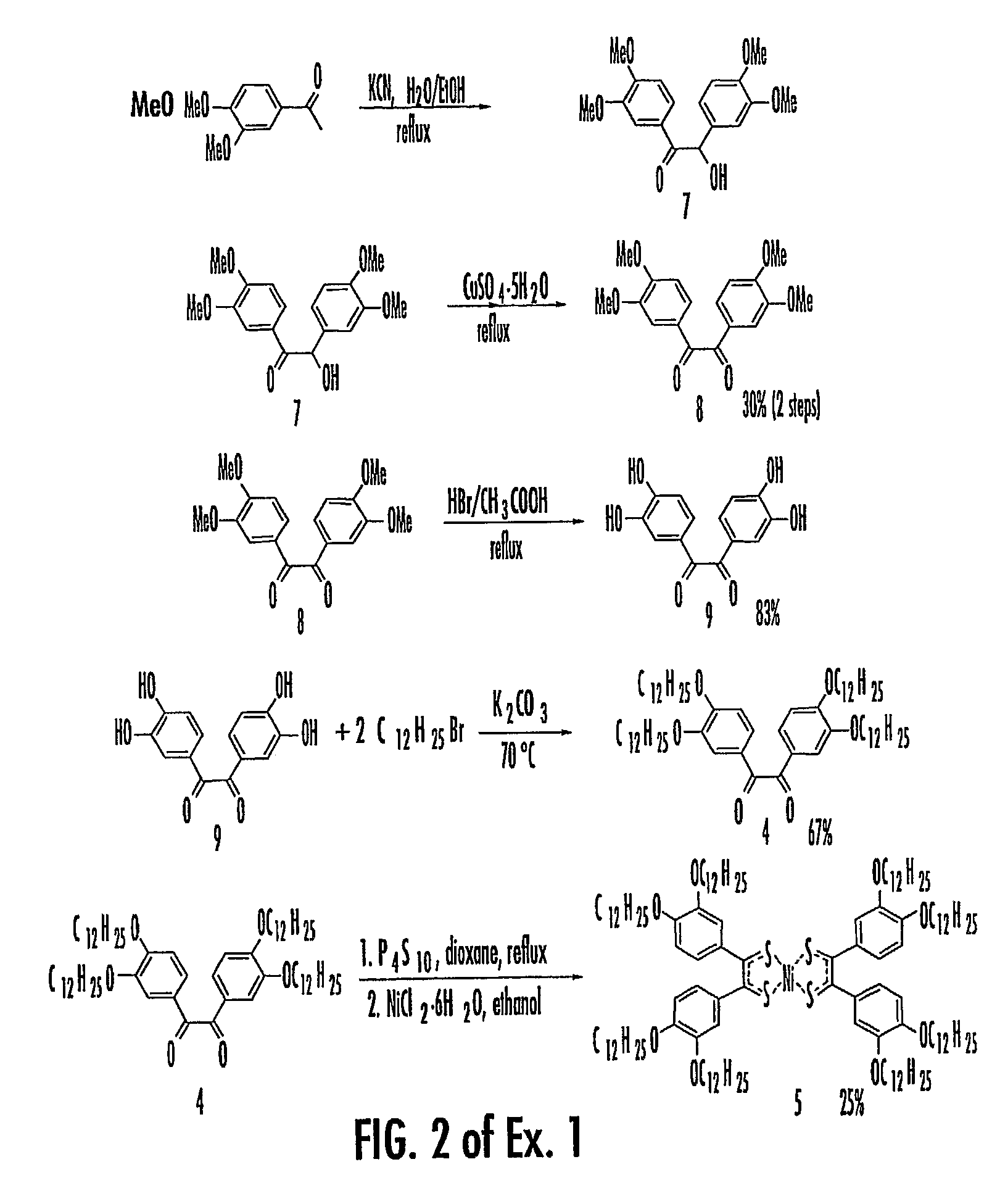
Georgia Tech inventors have created charge-transport materials; crystals, nanocrystals, liquid crystals, glasses, composites, polymers, co-polymers, and homopolymers including charge-transport materials; polymer layers including charge-transport materials, crystals, nanocrystals, liquid crystals, glasses, composites; and devices including charge-transport materials. One exemplary charge-transport material, among others, includes a transition-metal charge-transport material monomer having a structure of Formula I:M is selected from one of the following: nickel (II), palladium (II), platinum (II), cobalt (I), iridium (I), rhodium (I), copper (II), copper (III), silver (III), and gold (III). L and L′ can each be independently selected from one or more of the following groups: halogens, NR3, PR3, NCS, SCN, and CN.
- Potential for strong intermolecular overlap
- Low reorganization energies
- Tunability of redox potential
- Glass, crystal, liquid-crystal forming abilities
- Transport materials
- Light emiting diodes
- Field effect transistors
- Photovoltaic devices
- Sensors
- Phototransistors
- Radio frequency ID tags
- Detectors
Charge-transport molecular and polymeric materials are semiconducting materials in which charges can migrate under the influence of an electric field. These charges may be present due to doping with oxidizing or reducing agents, so that some fraction of the transport molecules or polymer repeat units is present as radical cations or anions. More usually, charges are introduced by injection from another material under the influence of an electric field. Charge-transport materials may be classified into hole- and electron-transport materials. In a hole-transport material, electrons are removed, either by doping or injection, from a filled manifold of orbitals to give positively charged molecules or polymer repeat units. Transport takes place by electron-transfer between a molecule or polymer repeat unit and the corresponding radical cation; this can be regarded as movement of a positive charge (hole) in the opposite direction to this electronic motion. In an electron-transport material, extra electrons are added, either by doping or injection; here, the transport process includes electron-transfer from the radical anion of a molecule or polymer repeat unit to the corresponding neutral species. In addition, some material—ambi-polar materials—may transport both holes and electrons.