Active pharmaceutical ingredients that have poor aqueous solubility have limited applications for use in existing dissolving microneedle drug delivery technologies. To overcome the poor drug solubility, the current approach is to use organic solvents for microneedle patch casting, which can complicate the manufacturing process with long evaporation steps and destabilization of the bioactive materials. Besides the intrinsic instability of biomolecules, liquid formulations needed for their casting pose an additional risk of degradation. Another limitation is the controlled or extended release of protein antigens, which needs sophisticated microneedle designs or harsh encapsulation conditions that all have a detrimental effect on active ingredients.
Melting microneedles offer solutions as well as many benefits
These melting microneedles are made of hydrophobic matrices of waxes that melt once inserted into skin and body temperature is reached. The drug or vaccine within the molten matrix diffuses out into the surrounding interstitial fluid based on its intrinsic aqueous solubility, viscosity of the molten wax, and rate of wax clearance from the skin. This approach can be used with poorly soluble drugs as well as water-soluble drugs that can be dispersed into the molten wax prior to casting. This eliminates the need for organic solvents in the first cast, improving stabilization for the loaded drug or vaccine. It also shortens the manufacturing time, eliminates the need to maintain a cold chain for storage and transportation, and reduces the ultimate cost of the microneedle patch. These safe hydrophobic matrices are inexpensive and enable control of the drug release timing. An expected shorter microneedle patch wear time will be more appealing to users and may result in better compliance and optimal delivery of the loaded drug.
- Stabilizing: This technology enables poorly soluble as well as water-soluble drugs to be dispersed, eliminating the need for solvents which translates to improved stabilization.
- Faster production: Unlike dissolving microneedles that often take days to dry their aqueous or organic-based formulations, these waxes require no further drying steps since the solidification of the microneedles is instantaneous upon cooling.
- Simplifies handling: Excluding water from the drug/vaccine casting process altogether further improves stability, allowing extended storage time outside the cold chain.
- Reduces costs: Simplified production and handling—in addition to the use of inexpensive hydrophobic matrices—reduces costs.
- Safe: Hydrophobic matrices used in the manufacturing of the melting microneedles have a well-documented safety record with many years of usage.
- Controllable delivery: The hydrophobic nature and the wide variety of wax composition enables tailoring of the extended-release profile for drugs, vaccines, or hormones, meeting specific desirable release profiles.
- Shorter wear time: Unlike dissolving microneedle drug delivery that can be delayed because of limited interstitial fluid at the site of microneedle patch application, melting microneedle patch wear time can be much shorter as it only depends on melting, which can be very fast. This feature will be more appealing to users and could translate to better compliance and assured delivery of the entire loaded dose per patch.
- Stronger immune response: Extended delivery of an inactivated antigen enabled through loading into melting microneedles has the potential to present antigen over a prolonged time period and generate a stronger immune response to the same dose.
- Drug delivery applications to humans and animals
- Hormonal delivery that requires an extended period of daily administration, such as contraceptives or hormone replacement therapies
- Extended-release vaccines
- Poorly soluble drugs
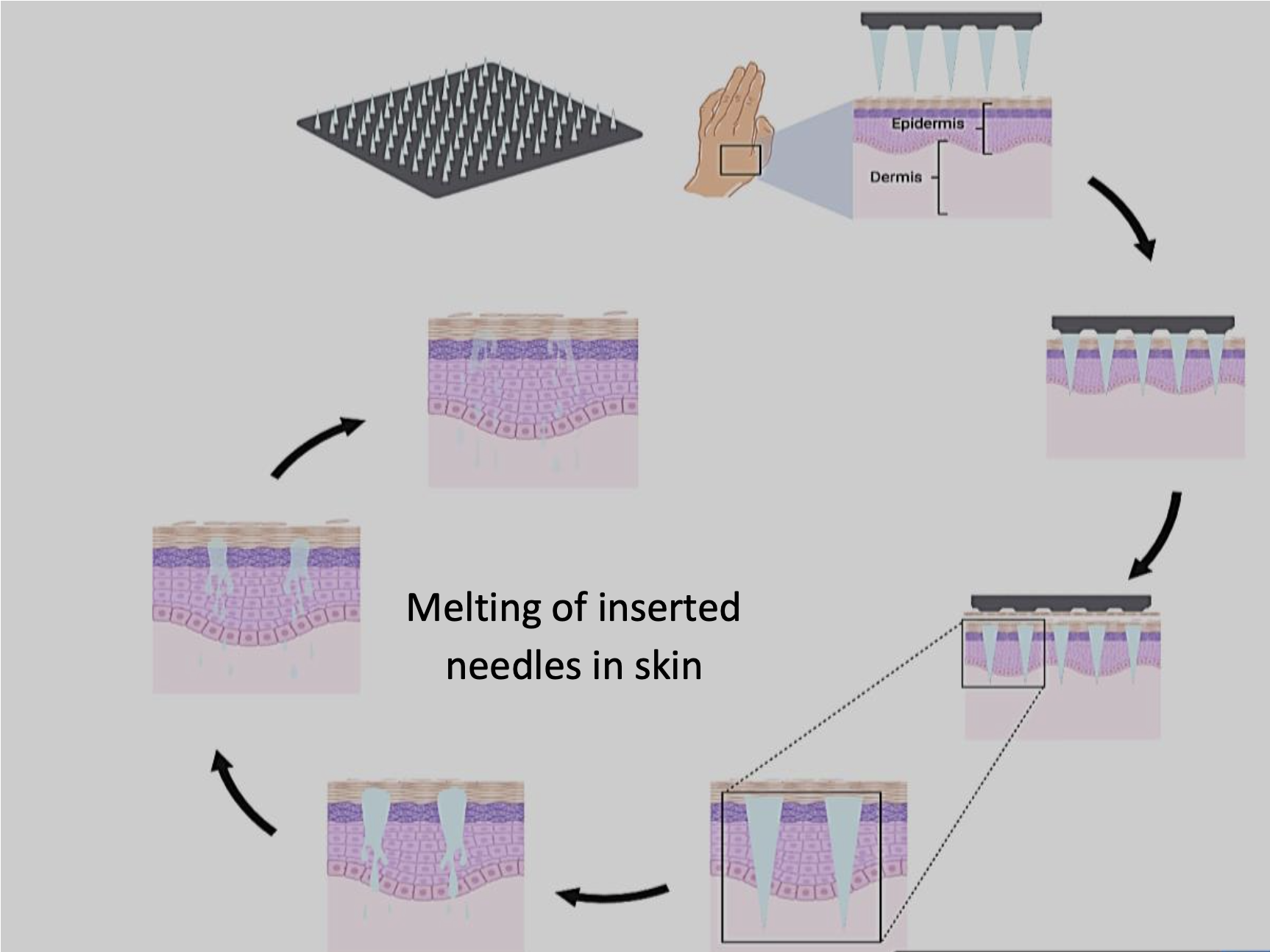
Diagram showing the mechanism by which melting microneedles deliver their payloads in skin.
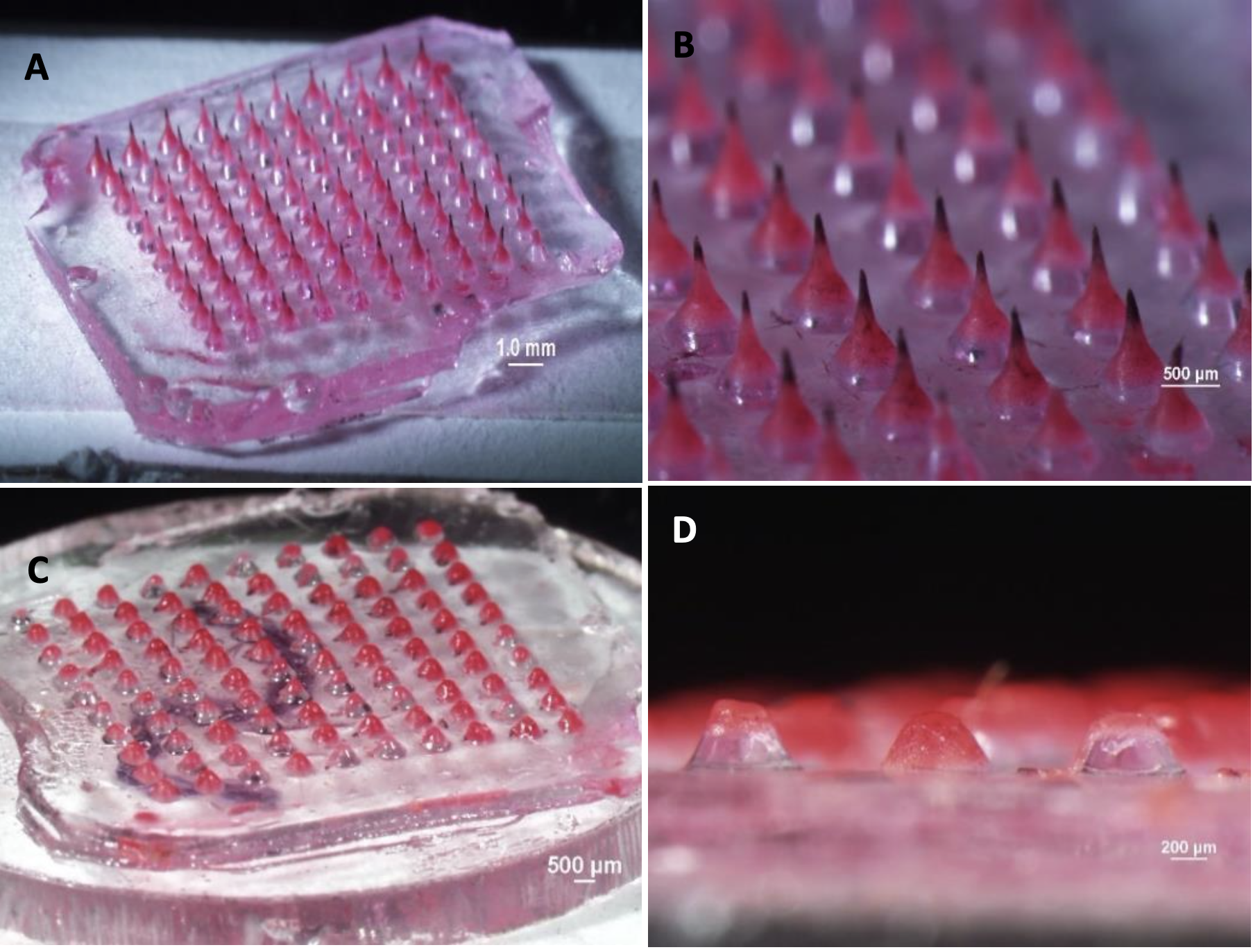
Melting microneedle patches before (A & B) and after (C & D) application to porcine skin ex vivo equilibrated to 32°C.
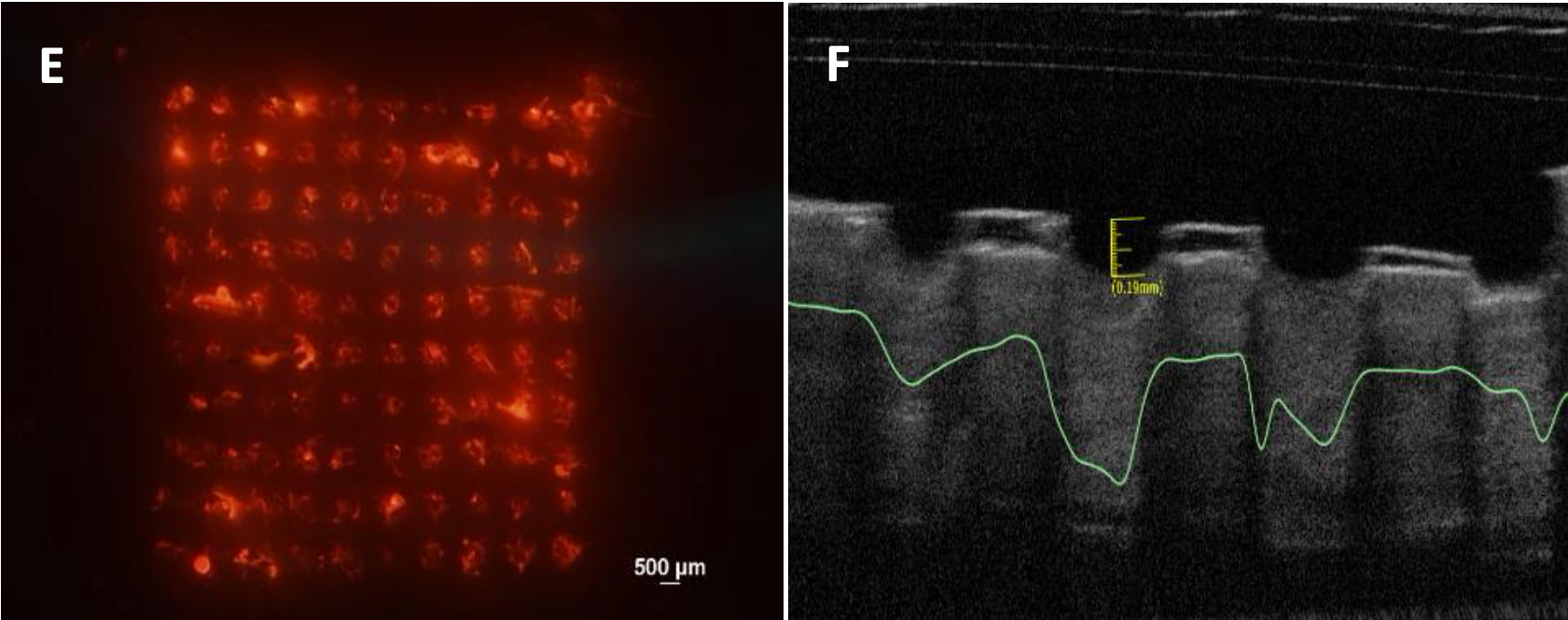
E – Fluorescent image of porcine skin showing the detachment of the needles from the supporting backing and their deposition in the skin; F – Optical coherence tomography image showing in situ skin insertion of the needles in porcine skin ex vivo.
