Georgia Tech inventors have developed a chemical storage system that provides reversible hydrogen storage and release at ultra-high capacity, density, speed, and ease, providing for low energy cost. The technology is based on a foldable polymer backbone that allows reversible uptake/storage/release of hydrogen fuel in response to thermal, chemical, mechanical, magnetic, electrical, or light stimuli. The storage system uses a natural or synthetic polymer with highly compact folding properties and chemically linked hydrogen-affine molecular complexes to provide an ultra-high surface area for hydrogen storage.
- Increased storage density — backbone offers high surface area for hydrogen storage
- Fast and easy — polymer readily folds and unfolds for hydrogen storage and fast release in response to actuation
- Suitable for packaging — uses compliant polymers as a matrix to assume any form factor
- Hydrogen storage
- Automotive fuel cells
- Energy production
- Distributed power generation
Conventional methods for hydrogen storage, such as compression and liquefaction, are not readily transferable to automotive or other portable applications. Improved hydrogen storage media and methods will enable greater use of alternative energy sources and reduce the reliance on hydrocarbon-based fuels. There is a need for a technology to provide a highly efficient method for hydrogen storage to make energy storage on board of vehicle economically feasible.
See other hydrogen fuel–related technologies by Dr. Fedorov. To see his entire portfolio of available technologies, click here.
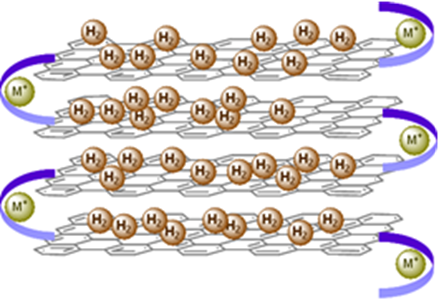
Figure 1. Graphite-mimetic stacked polymer for interlayer hydrogen storage with metal ion-mediated folding (Credit: Charles Liotta & Charles Eckert).
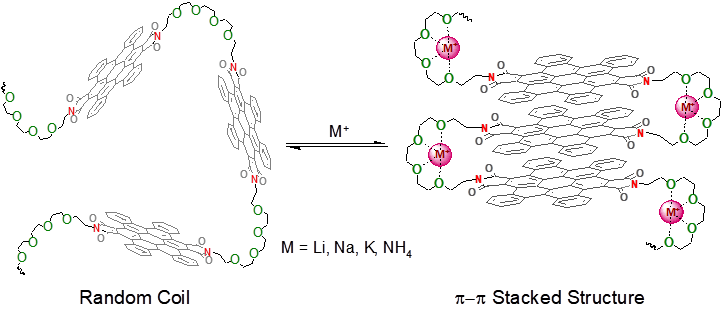
Figure 2. Metal ion mediated folding of perylenebisimid-oligo(oxyethylene) polymers leads to efficient self-stacking at the separation for optimum hydrogen storage (Credit: Charles Liotta & Charles Eckert).
