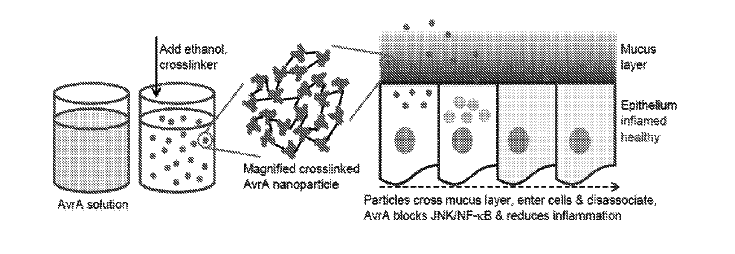
Julie Champion, Lina Herrera Estrada, and Zachary Wells from the School of Chemical and Biomolecular Engineering at Georgia Tech have developed a new drug delivery system by creating delivery nanoparticles out of the therapeutic protein to be delivered. The particles are made by conjugating recombinant proteins using disulfide bonds. Having the therapeutic protein be its own delivery method maximizes the amount of protein in the nanoparticle and preserves bioactivity. It also protects the protein by reducing the amount of exposed surface area. The nanoparticles are made by desolvation, and subsequently crosslinking the proteins together to stabilize the particles. Researchers have shown the anti-inflammatory effects of fabricated particles from Salmonella protein AvrA in vitro and in vivo, showed protective effects from an engineered influenza vaccine in mice, and witnessed high levels of cell death in vitro using a Yersinia protein for breast cancer applications using this delivery system.
- Low cost and effective production method
- Maintains protein structure and enzymatic activity and maximizes the amount of protein to be delivered
- Preserves bioactivity
- Reduces amount of exposed surface area and increases absorption by target cells
- Higher affinity and specificity
- Able to rapidly convert and amplify target molecules into products
- The delivery of any protein into cells
- Anti-inflammatory proteins
- Vaccination
- Breast cancer applications
- Potential for antibodies, enzymes, inhibitory proteins, etc.
Protein drugs and enzyme therapeutics are becoming a critical research focus in healthcare. Enzyme therapeutics offer benefits over traditional small molecule drugs, such as higher affinity and specificity and the ability to rapidly convert and amplify target molecules into therapeutic products. However, the delivery of protein drugs have challenges not seen by traditional small molecule drug delivery methods. The large size and complex structures of proteins make them sensitive to the environment and incapable of surviving clearance processes in blood and tissue. Currently, small molecule drug delivery involves encapsulation by polymeric or liposomal carriers, but proteins are too large to be absorbed by cells. Therefore, a delivery system that maintains enzymatic activity and increases absorption by target cells is needed.