This invention describes a new drug delivery system for improving cancer treatment via the local and sustained delivery of antibodies targeting immune checkpoints. It utilizes a local intradermal injection of in situ crosslinked hydrogel to tumor-draining lymph nodes in order to deliver immune checkpoint blockade antibodies and promote activation of t cells so that they can infiltrate the tumor microenvironment. Specifically, the Georgia Tech research team’s method mixes synthesized thiolated thermosensitive polymers with maleimide functionalized polyethylene glycol to make the hydrogel. The hydrogel is then combined with the antibody of interest before delivery.
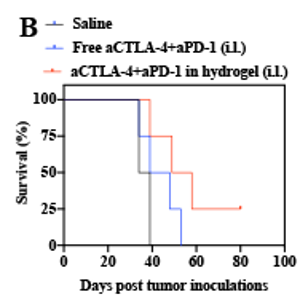
In early experiments, this technology demonstrated reduced tumor growth, improved survival rates, and minimal liver toxicity. By promoting the differentiation, proliferation, and activation of tumor-specific t cells, the crosslinked hydrogel leverages the body’s immune system to augment the effects of antitumor treatments.
- Versatile: Can integrate with all types of immune checkpoint blockade antibodies and respond to various types of cancers
- Improved tolerability via local delivery: Limits side effects on nonmalignant tissues and organs—a challenge of other immunotherapies
- Innovative: Leverages the body’s natural immune response to reduce the likelihood of cancer recurrence
- Anticancer treatments
Immunotherapy has become a widely popular method for treating cancer as it leverages the body’s immune system to target and kill cancer cells naturally. Immunotherapies are usually administered systemically; however, this route often leads to adverse effects on nonmalignant tissues and organs and limits the effectiveness of the treatment on the tumor site. Georgia Tech’s crosslinked hydrogel delivery method meets the need for innovative drug delivery systems for immune checkpoint blockade antibodies, which could serve to improve the viability of immunotherapy as an alternative to chemical drug treatment.
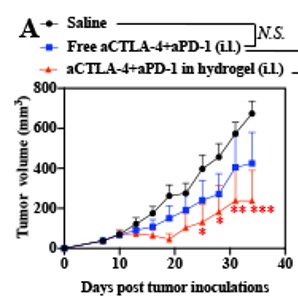
These figures show a decrease in tumor volume (Figure A) and improved survival (Figure B) via the crosslinked hydrogel.