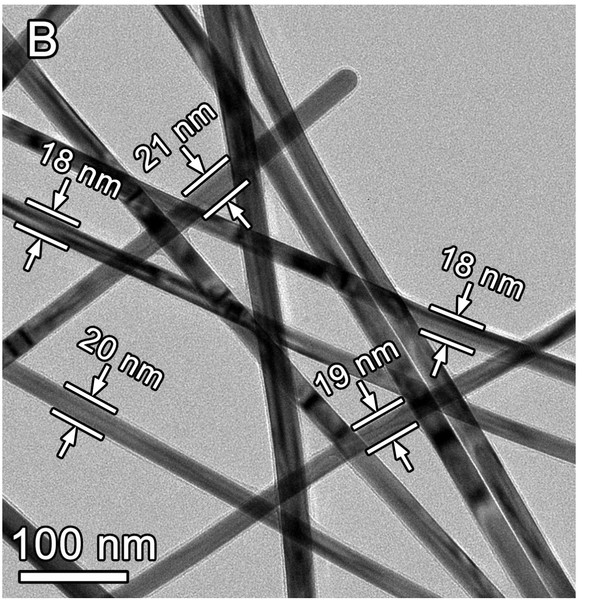
Younan Xia, and Robson Rosa da Silva from the School of Biomedical Engineering at Georgia Tech have created a simple one-pot method to produce Ag nanowires that are less than 20 nm in diameter with aspect ratios of more than 1000. This is accomplished by confining the nanowire growth in the lateral direction by adding bromide ions and poly(vinylpyrrolidone) (PVP) with a high molecular weight which acts to “cap” the side faces of the wires while introducing silver nitrate slowly into the reaction vessel. The nanowires had a penta-twinned structure and were obtained in a high yield (90%) within 35 minutes. Specifically, the controlled growth of the Ag nanowires was accomplished by introducing bromide ions from NaBr or KBr and high molecular weight (1,300,000) PVP to act as a capping agent.
Through the optimization of the ratio of the capping agent and the silver nitrate, the researchers successfully demonstrated controlled nanowire growth in the longitudinal direction only. Here, the bromide ions bind to the silver ions to form silver bromide (AgBr). This succeeds in slowing the overall reaction speed by locking up any free Ag ions. This slower reaction rate leads to the preferential formation of penta-twinned, small decahedral Ag seeds that grow into penta-twinned nanowires. The nanowires have significantly reduced localized surface plasmon resonance (LSPR), leading to enhanced transparency in the visible region of the spectrum. This transparency, coupled with their good electrical conductivity and mechanical flexibility could make them ideal transparent conductive electrodes.
- Produces Ag nanowires less than 20 nm in diameter, with high aspect ratios (over 1000), and in high yield (>85%)
- One pot, quick (< 35 min) synthetic process that is performed under ambient pressure
- Great mechanical flexibility and can be bent at acute angles without breaking
- The transverse localized surface plasmon resonance peak of the Ag was below 400, making them highly transparent in the visible spectrum
Ultrathin Ag nanowires have enormous potential as the conductive component in touch screens, LCD displays, optoelectronics such as LEDs and photovoltaics. Additionally, Ag nanowires show promise for interconnects, conductive ink, IR shielding, and transparent heaters. They can also be used in a range of biological or chemical sensing applications.
Silver (Ag) nanowires are generating intense interest as conductive transparent electrodes to replace ITO (indium tin oxide) which is expensive and in short supply. However, in order to realize this potential, the transparency of Ag nanowires in the visible spectrum needs to be improved. This can be accomplished by increasing the aspect ratio while decreasing the diameter of the nanowires involved, but this has proven to be difficult and thus the promise of Ag nanowires has remained elusive until now.