These tissue-selective histone deacetylases inhibitors (HDACi) suppress the growth of transformed/cancerous cells and display tissue-targeted anticancer activities. These compounds contain macrolide moieties, which accumulate in resident macrophage-enriched organs, including the lungs, liver, and spleen, and in tumors—particularly breast tumors.
In mice experiments, representative compounds inhibited the growth of primary mammary tumors and protected the lung tissues from tumor infiltration. The compound accumulated within the tumor nodules in the liver and the lungs at levels that are 50 to 90 folds higher than in healthy lungs and liver. This demonstrates the possibility of successful delivery of treatment to tumor nodules, potentially rendering these compounds more effective than currently available HDACi agents.
Additionally, Georgia Tech researchers identified other cohorts of HDACi that target liver tissue as a new class of anti-hepatocellular carcinoma (HCC) agents. Tests showed that this class of macrolide-based HDACi, which are selective for sub-class I HDACs, preferentially accumulated in the liver tissue and robustly suppressed tumor growths in an orthotopic model of HCC. The liver tissue-selective accumulation property of these compounds may give them a unique advantage over most current HDACi. Thus, these compounds have the potential to positively impact HCC therapy.
See also #8373, "Small Molecule Glycosylated Histone Deacetylase Inhibitors (HDACi)”
- Tissue-targeted activity: Appended macrocyclic skeletons provide selective tissue distribution.
- Potent: HDAC inhibitors display stronger in vivo anticancer activity against solid tumors compared to current technology.
- Lower toxicity: Mice in preclinical experiments did not exhibit significant weight loss, pointing to potentially lower toxicity of these compounds.
- On-target effects: The HDAC sub-class I selective compounds induced dose-dependent histone H4 hyperacetylation without perturbation of tubulin acetylation status and G0/G1 cell cycle arrest.
- Liver cancer
- Lung cancer
- Breast cancer (regardless of hormone expression status)
- Idiopathic pulmonary fibrosis (IPF)
A need exists for a solution to improve the safety and toxicity of current anticancer drugs to influence cancer treatment and management.
Lung cancer remains one of the top causes of cancer deaths in the United States. Several pharmacological strategies (chemotherapy, immunotherapy, and druggable target identification) have emerged for the treatment of metastatic diseases, including lung cancer. Despite this progress, chemotherapy remains the primary treatment choice for most cancer cases. However, most chemotherapy elicits severe toxicities and other undesirable side effects.
Liver cancers are among the leading causes of global cancer deaths. The current therapy options for liver cancers, including HCC, have afforded limited benefit to patients who have an advanced disease state.
HDAC inhibition is an exciting, clinically validated cancer therapy strategy. As of 2020, five HDACi have been clinically approved but all for the treatment of hematologic malignancies. Safer and more effective HDACi are needed to target solid tumors.
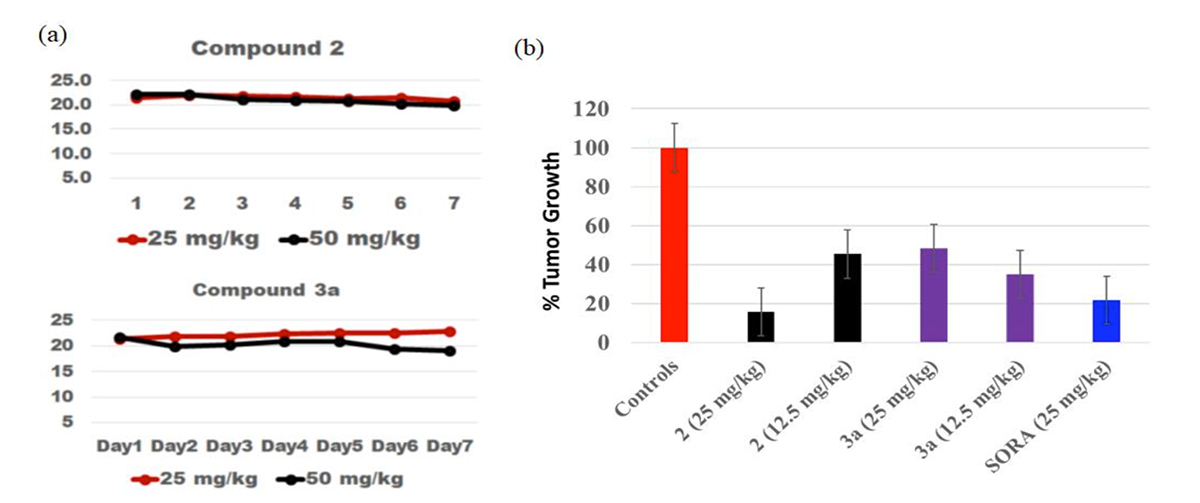
Preclinical in vivo efficacy of two of the Georgia Tech macrolide-based HDACi compounds (2 and 3a). The compounds did not lead to decreased weight in the rodent experiments over a period of 7 days (a), while suppressing tumor growth orthotopic models (b).
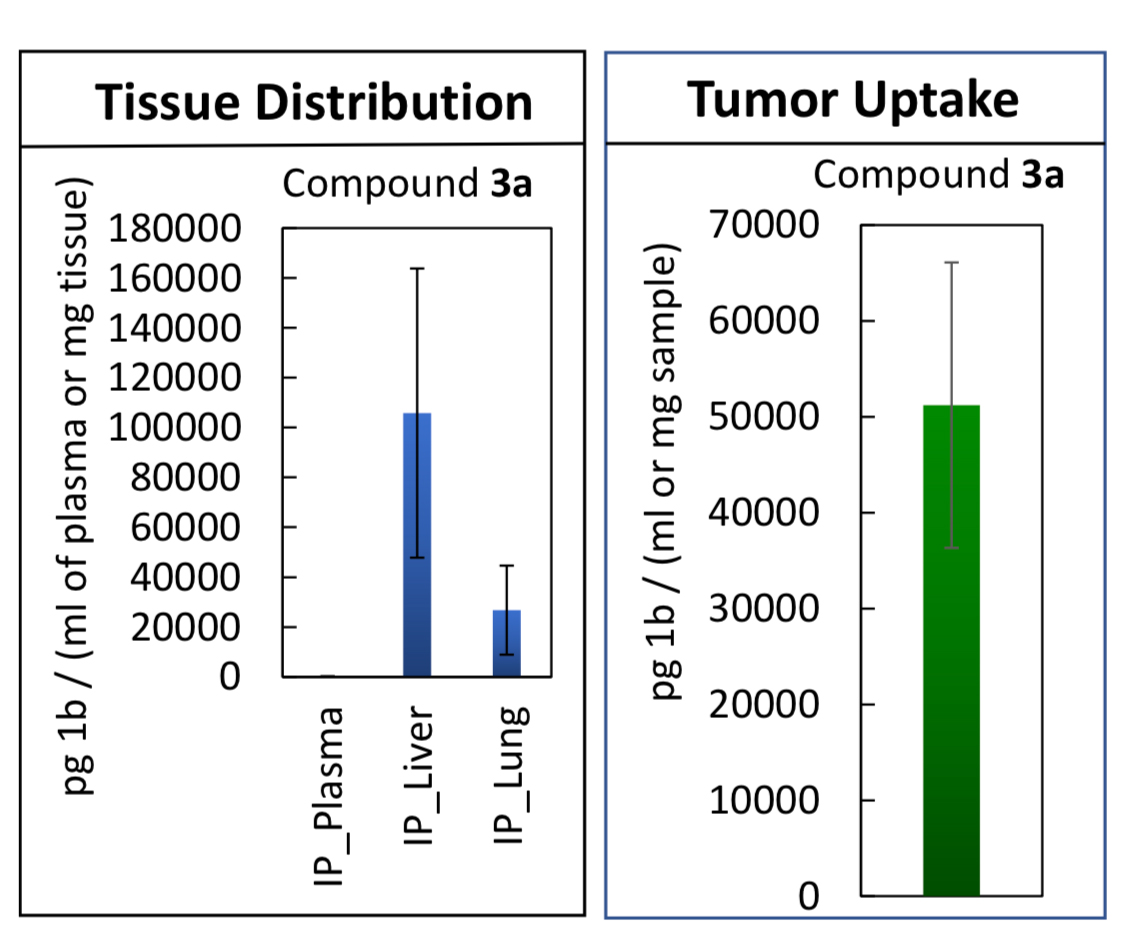
Analysis of tissue distribution and tumor uptake of one Georgia Tech compound demonstrating accumulation on liver and lung tissues and robust tumor uptake.
