Novel pharmaceutical compounds have been identified by researchers at Georgia Tech that are capable of inhibiting histone lysine demethylase (KDM) enzymes, globally changing histone modification profiles, and targeting specific tumors.
This research provides new insights into the mechanism of the antiproliferative activities of deferiprone (DFP), a hydroxypyridinone-derived iron chelator currently in clinical use for iron chelation therapy. The data show that DFP derives its antiproliferative activity primarily by inhibiting a subset of KDMs. Its docked orientations at KDM-active sites permit modifications that have enabled identification of a cohort of new DFP-based antiproliferative agents. These agents display selective cytotoxicity against specific cell lines, with one DFP-based KDM inhibitor compound displaying a tumor-selective cytotoxicity potency enhancement as high as 65-fold relative to DFP against the triple negative breast cancer (TNBC) cell lines tested.
The novel DFP-based KDM inhibitors, with composition-of-matter patent protection, have the potential to be effective tumor-selective cancer therapeutics, particularly for TNBC.
- Selective: Can be used to target specific types of tumors
- Proven efficacy in vitro: Can be used to inhibit proliferation of cells (e.g., cancer or tumor cells), reducing tumor burden, inhibiting tumor growth, or both
- Enhanced potency: Displayed a 65-fold increase in cytotoxic potency against tested triple negative breast cancer cell lines
- Primary application:
- Breast cancer, especially TNBC
- Other applications:
- Liver cancer
- Lung cancer
- Prostate cancer
KDMs play a broad and significant role in the proliferation of cancer cells. In fact, specific members have been identified as essential in supporting the growth of several types of cancers. This research identifies several new DFP-based KDM inhibitors that alter the velocity of HP1-mediated heterochromatin gene repression to slow the progression and growth of many types of cancer cells.
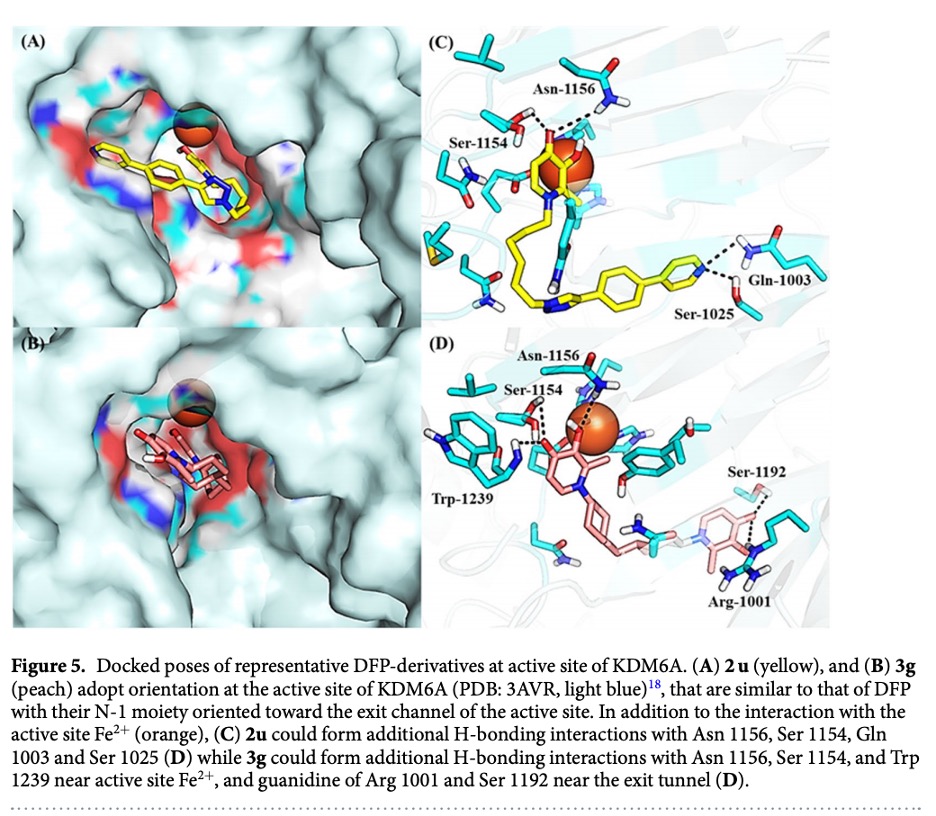
Because high levels of iron are essential for tumor cell growth, the antiproliferative effect of DFP has been largely attributed to its iron chelation activity. Using molecular docking, it was determined that DFP adopts docked orientations in which it strongly chelates Fe2+ at the active site of KDM6A and is oriented in a way that may permit modifications to enhance binding affinity for a subset of KDMs. The docked poses adopted by DFP at KDM-active sites also permitted modifications that enabled identification of a cohort of new DFP-based antiproliferative agents, which displayed selective cytotoxicity against a TNBC cell line.
DFP was found to gain access to and form a bidentate chelate with the Fe2+ at the active site of a subset of KDMs. The DFP-KDM interaction was further stabilized by H-bonding between the oxygen moieties of DFP and key residues within the enzymes active sites. This in silico observation implicates DFP as a potential inhibitor of the demethylase activity of six KDMs (2A, 2B, 5C, 6A, 7A, and 7B).
This new class of molecules caused a delay in heterochromatin gene repression that was not seen in the parent DFP, and a representative compound (2j) potently suppressed the growth of T47D and MDA-MBMB-231 in murine xenograft models.