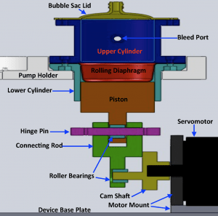
Inventors at Georgia Tech and Emory have invented a pediatric-specific CRRT device that increases safety, improves fluid accuracy, reduces extracorporeal blood volume, and eliminates other problems associated with the current practice of using adult CRRT devices for pediatric care. The unique mechanical design consists of a closed loop system that provides a means to safely control the blood that is drawn, filtered, supplemented, and returned to the patient; and a fluid balance system that resolves the inaccuracy between the amount of ultra-filtrated produced and replacement fluid delivered compared to currently available devices. An offset loop to adjust the balanced fluid exchange allows clinicians to impose a positive or negative state of fluid balance. The touch screen interface is programmed to communicate and be compatible with electronic medical records, and has a number of additional features. Its size addresses the problem of restricted bedside space. The device has been designed to be quieter through the elimination of operating valves, which may be particularly beneficial for use with neonates and small children.
- Pediatric-friendly- elimination of existing application of adult CRRT devices for pediatric care
- Improved accuracy- novel pump and control system design complete with offset loop and fluid balance system
- Versatile- easily integrated with other extracorporeal therapies
- User-friendly interface- reduced size of device in comparison to current therapy devices
- Safer- reduced risk of infection, bradykinin release syndrome, and transfusion reactions by reducing extracorporeal volume, thus reducing number of blood exposures
- Treatment for CRRT for neonates and children with acute kidney injury
Approximately 5000 children annually in the U. S. require continuous renal replacement therapy (CRRT). A form of adjunct support is often vital to provide fluid and electrolyte clearance until native renal function improves for children with acute to moderate kidney failure. Currently, there are no CRRT devices approved for pediatric use. Instead, devices used in adults must be adapted for use in children. As these devices are not designed for children, they may not deliver proper pediatric care. Currently available devices often result in (1) an imbalance between the amounts of ultra-filtrate produced and replacement fluid delivered, (2) inaccurate pump delivery leading to dehydration, and (3) reduced extracorporeal volume leading to additional blood exposures, which puts the patient at risk of infection, antibody development, bradykinin release syndrome, and other transfusion reactions.