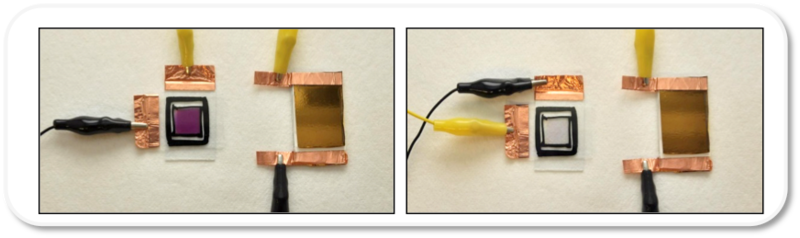
John R. Reynolds, James F. Ponder Jr., Anna M. Osterholm, and Rayford H. Bulloch from the School of Chemistry and Biochemistry at Georgia Tech have developed a soluble electroactive polymer using solvent-resistant conjugated polymers. Initially in their ester form, the polymers are soluble in organic solvents but can be rendered water-soluble through defunctionalization of the alkoxy-chains with an organic or metal hydroxide. It also is highly soluble in other polar solvents, such as dimethylformamide (DMF) but is insoluble in relatively non-polar solvents (e.g., chloroform).
The water-soluble polyelectrolyte form of these polymers can be cast into thin films and further treated with acid to resist solvents, which makes them compatible and highly electroactive in both organic and aqueous electrolyte systems for redox switching. These films demonstrate exceptionally rapid switching compared to similar polymers using typical aliphatic side chains in both organic (LiBTI/PC) and aqueous (LiBTI/H2O or NaCl/H2O) electrolyte solutions.
- Environmentally friendly: Aqueous/Organic processing is safer than the aromatic hydrocarbon and chlorinated solvents typically used.
- Higher levels of electric power: Films made with this technology have significantly faster redox switching rates of up to 10V/s.
- Versatile: Various electrolyte systems have been successfully tested, including organic, aqueous, the spinal fluid analog Ringer’s solution, and even commercially available sports drinks.
- Fuel cells
- Charge storage (including solar)
- Supercapacitors
- Electrochromism (e.g., smart glass)
- Thermoelectrics (e.g., refrigeration, power generation)
- Bioelectronics (e.g., field effect transistors)
This technology is a conductive polymer with low oxidation potential and high charge capacity that does not redissolve in solvent or electrolyte. Electroactive polymers are desirable for electrochemical supercapacitor applications as they are psuedocapacitive, switch rapidly between redox states, and are efficient ion transporters. The researchers’ goal was to obtain a solution-processed polymer that retains the conducting and stability properties of PEDOT (poly[3,4-ethylenedioxythiophene]), with improved solubility and broader electroactive window.