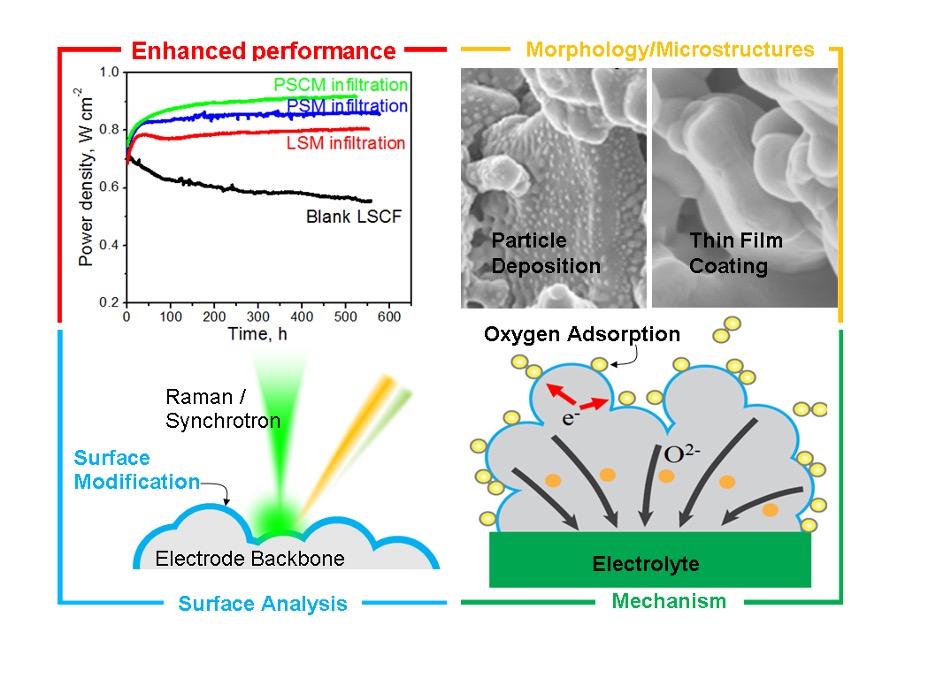
These two novel catalyst conformal coatings for solid oxide fuel cells have dramatically enhanced both stability and electro-catalytic activity on LSCF (La0.6 Sr0.4 Co0.2 Fe0.8 O3−δ) porous cathodes. The success of these Georgia Tech–developed active Mn-based catalyst coatings has been demonstrated in symmetrical and anode-supported cells.
Using dense, conformal, thin-film coatings of PSM (Pr0.75 Sr0.2 MnO3−δ) and PSCM (PrSrCoMnO6-δ) on state-of-the-art LSCF-based cathodes produces excellent electro-catalysts for surface modification of SOFC cathodes.
In addition to enabling lower operating temperatures (700°C or lower), which is critical for commercial production, the simple and efficient one-step process can be readily implemented into typical SOFC fabrication. The catalyst coatings are also very thin (10 to 50 nm), potentially enabling other catalytically active materials that are typically too expensive to use to become economically feasible.
- Enhances activity and stability: Electro-catalytic activity and stability were enhanced in symmetrical and anode-supported cells.
- Eliminates technical hurdles: By reducing operating temperatures to 700°C or lower, this process makes SOFCs more commercially feasible.
- Efficient: The efficient one-step infiltration process enables implementation for typical SOFC fabrication.
- Cost-effective: Because the film is very thin, other more expensive and desirable catalytically active materials may become economically possible.
- Solid oxide fuel cells
- Oxygen separation membranes
- Membrane reactors that involve oxygen reduction
- Energy conversion
- Reversible fuel cells for energy storage
- Distributed power generation
Intensive research has been conducted in the field of fuel cell technologies in the search for a clean and sustainable energy supply that has low environmental impact. Fuel cells provide high conversion efficiency through direct conversion of chemical fuels to electricity, with solid oxide fuel cells having the greatest potential due to their use of low-cost ceramic materials and extremely high electrical efficiencies. SOFCs can use a wide variety of fuels, including hydrogen and hydrocarbons, coal gas, and bio-derived fuels. However, significant technical challenges have inhibited commercialization of SOFCs because they require high operating temperatures (generally above 800°C), which requires the use of expensive materials and creates engineering challenges.
This technology’s enhancement of cathode activity represents a vital step toward successful reduction in operating temperature since the resistance to oxygen reduction reaction contributes the most to energy loss in existing SOFCs, and even more so at lower temperatures.