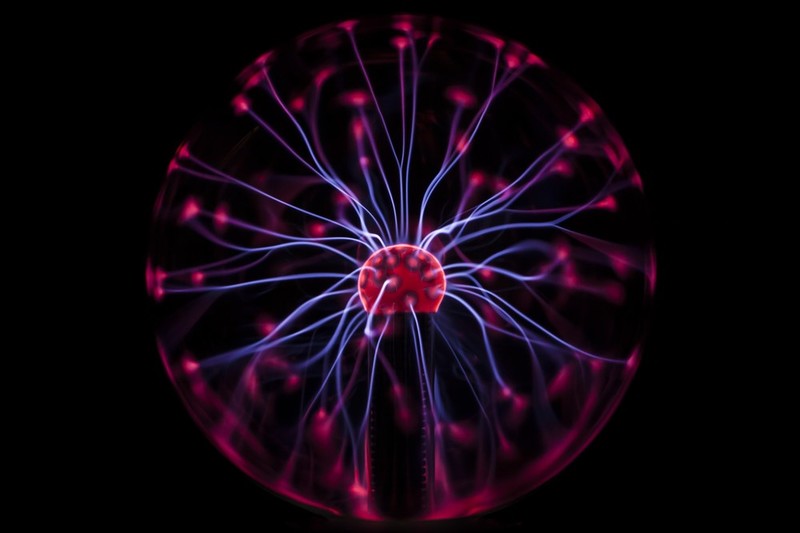
Georgia Tech inventors have developed a method to generate free radicals using electrochemical means. A small electrode of specific dimensions and shape is inserted into the liquid reaction medium and then energized at 400 mA and 1.5 kV. This causes electrons to be stripped from molecules near the electrode, forming a small plasma. The main difference between this and other free radical generation techniques is:
i) This process can be carried out at room temperature and
ii) The free radical source can be turned “on” and “off” by varying the electrode potential.
This provides a large degree of control over the concentration of free radicals and thus the observed reaction rate.
- Controllable source of free radicals
- Ability to concentrate free radicals in one location
- Replaces free radical initiators
- Proven efficacy in polyene polymerization reactions
- Initiating free radical reactions- ability to produce radicals at highly localized regions, which allows the source of controllable free radicals for polymerization reactions and the treatment of effluent from paper recycling facilities by polymerization of ink solutions containing alkene bonds, allowing it to be filtered
Plasmas can be created by super heating a gas or liquid, or by subjecting it to a strong electromagnetic field. Once formed, the plasma is rich in reactive species such as free radicals. Free radicals are a molecule or atom with a single unpaired electron in its outer shell. Chemical reactions which are propagated by free radicals comprise one of the three general types of chemical reaction (the other two being anionic and cationic). The most common ways to generate free radicals is by using radical initiators such as peroxides, or by photochemical means. However, since free radicals are so reactive, the reaction conditions have to be optimized such that only a certain concentration of free radicals is present otherwise, the reaction will self-terminate when two radicals meet each other.