This method for engineering enzymes yields a heterologous thioesterase (TE) that improves medium-chain fatty acid (MCFA) production during chemical and biopolymer synthesis. MCFAs are key intermediates in the synthesis of medium-chain chemicals, including dicarboxylic and hydroxy acids, useful in polymer production. They are not naturally biosynthesized by microbes and their production is hindered by the inefficient expression of plant and bacterial TEs with broad fatty acid profiles.
Georgia Tech researchers have engineered a TE enzyme to interact with endogenous acyl carrier protein (ACP) proteins to achieve a three-fold MCFA yield in E coli. This innovation demonstrates that engineering the interface of heterologous enzymes to better couple with endogenous host enzymes may be a useful strategy for improving the microbial production of chemicals that require the expression of heterologous enzymes. Further, this work sets the stage for sensor-guided engineering of MCFA-producing microbes.
- High performance: Increases the yield of MCFAs during chemical and biopolymer synthesis
- Enabling: Improves the microbial production of chemicals that require the expression of heterologous enzymes
- Adaptable: Potentially applies to methods of engineering other heterologous enzymes to interact with endogenous proteins in order to produce molecules of interest in a bacterial host
This innovation could improve the production of biopolymers. In addition, MCFAs are used as herbicides, antimicrobials, and intermediates for lubricant synthesis.
MCFAs have uses as antimicrobials and emulsifying agents, and their derivatization using chain-tailoring enzymes produces an array of chemicals including alkenes, alcohols, and ketones. Because of the inefficient expression of plant enzymes in bacteria and the broad fatty acid profile of most bacterial TEs, there is a need to engineer TEs with improved MCFA production.
In this work, Georgia Tech researchers optimize the interface between E. coli ACP and the medium-chain acyl-ACP thioesterase from Acinetobacter baylyi (AbTE). They docked E. coli ACP with the endogenous E. coli TE TesA and identified potential contact residues involved in stabilizing the ACP-TesA interaction. The research team then mutated the equivalent positions in AbTE to the amino acids found in E. coli TesA and measured its fatty acid profile. They found that mutation of just two residues on the AbTE surface—G17 and A165 to arginines—improved MCFA titers more than three-fold when compared to the expression of AbTE wild type in E. coli. This is the first engineering of a heterologous thioesterase for improved MCFA production.
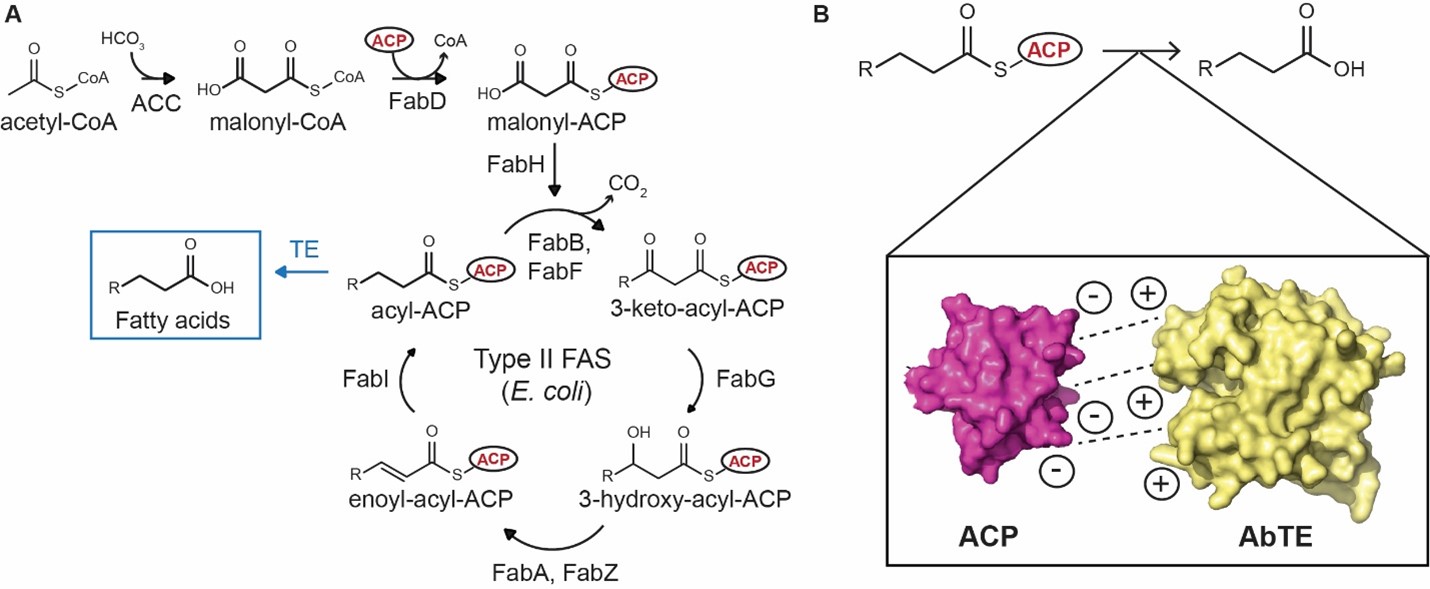
Medium-chain fatty acid biosynthesis in E. coli: A) E. coli Type II fatty acid synthase extends and reduces an acyl chain bound to acyl-carrier protein (ACP). Thioesterases (TEs) hydrolyze acyl-ACPs to free fatty acid of different chain lengths depending on their substrate specificity. B) Matching the surface interface between the native E. coli ACP and medium-chain heterologous TEs, such as Acinetobacter baylyi TE (AbTE) improves medium-chain fatty acid production in E. coli.
